
Group Information
- The Group
-
The liquid crystals group currently consists of four academic staff (Dr Rebecca Walker, Professor John Storey, Dr Alf Martinez-Felipe (School of Engineering) and Dr Peter Henderson ) supported by Technical Resources Officer Brian Paterson , and around five researchers.
The ALCG would like to recognise the immense contributions made by the group’s founder Professor Corrie T Imrie, who passed away suddenly in January 2025. Highly regarded by all at the University of Aberdeen and the international Liquid Crystal community, there are not enough words to describe the impact he had and, of course, the void his loss has left behind.

- Equipment
-
ALCG operates in a recently refurbished laboratory providing first-class facilities for chemical synthesis and characterisation. We have advanced organic synthesis (inc. Easymax 402 and Syrris automated synthesis systems), purification (inc. Agilent 1260 analytical and semi-prep HPLC, and Biotage® Selekt flash column chromatography systems) and analytical characterisation facilities (Bruker 80 (benchtop), 400 and 600 MHz NMR spectrometers, Thermo Nicolet Summit OA FTIR spectrometer, Waters XEVO G2 QTof high resolution mass spectrometer, Bellingham+Stanley ADP410 polarimeter).
We have a dedicated thermal analysis laboratory with technical support which includes two Mettler Toledo 821e differential scanning calorimeters (DSC 1 and DSC3), Mettler Toledo thermal gravimetric analyser equipped with a Hiden Instruments mass spectrometer, and a state-of-the-art microscopy suite with three polarised optical microscopes including a new Zeiss AxioImager A2m equipped with a Linkam THMS600 hot stage and Canon EOS 90D DSLR camera.
Just across the road, the School of Engineering hosts a series of new generation electrometers and analysers capable of determining dielectric relaxations and conductivity of liquid crystalline and inorganic materials, with Linkam stages and furnaces covering cryogenic to very high temperatures (~1400 °C). These include a Solartron 1260 frequency response analyser, a state-of-the-art AMETEK ModuLab© XM MTS station (capable of carrying out time domain and AC measurements with high and low impedance in the femto-ampere range), a Keysight E4980A meter (generating strong alternating electric fields), a Keithley 2450 source meter (to apply precise voltage biases of the liquid crystalline samples (in the range 20 mV-200 V)), and a Keysight (Agilent) 4191A Impedance Analyser (to study the dielectric response in the 1 MHz-1 GHz frequency range). These are assembled to operate simultaneously with a new Olympus polarised microscope, tailored to study phase behaviour under the application of UV radiation.
- Funding
-
Funders include:
The Carnegie Trust, EPSRC, The Royal Society, The Royal Society of Chemistry, The Royal Society of Edinburgh.





- Collaborations
-
We have ongoing collaborations with a number of research groups in Europe and further afield, including groups in Spain, Slovenia, Japan and the USA. We work extensivelty with the group of Professor Ewa Gorecka at the University of Warsaw in Poland. The University of Warsaw's Laboratory of Physicochemistry of Dielectrics and Magnetics houses state-of-the-art structural characterisation equipment to compliment our own, including multiple temperature controllable powder diffractometers and an atomic force microscope.
- Interested in joining the group?
-
If you're interested in joining the group as a PhD student, or establishing a collaboration, please email Dr Rebecca Walker (rebecca.walker@abdn.ac.uk).
Research Projects
- The twist-bend nematic phase
-
In a conventional nematic phase (N) the molecules, on average, lie along a common direction known as the director and their centres of mass are randomly distributed (a). When some, or all, of the molecules are chiral, the director twists in space forming a helicoid and this is referred to as the chiral nematic phase (N*) (b). A new nematic phase was discovered in 2011, the NTB phase, in which the molecules are achiral but the directors form a helix and are tilted with respect to the helical axis (c). The formation of chirality in the NTB phase is spontaneous and hence equal numbers of degenerate helices of opposite handedness are seen. This spontaneous emergence of chirality in systems composed of achiral molecules is of fundamental importance in both physical and biological sciences and thought to play a pivotal role in the origin of biological homochirality. In this context, the study of these liquid crystalline systems will greatly enhance our understanding of symmetry breaking in fluids.

In recent years, the NTB phase has become the hottest topic in liquid crystal science and the focus of intensive international research. The Aberdeen Liquid Crystals Group has published extensively on the NTB phase in dimeric liquid crystals, including the first example of an NTB phase driven by hydrogen bonding, the first NTB-N transition driven by photoisomerization, and shown how mixing achiral components can lead to spontaneous structural chirality. In collaboration with research groups in the USA, we have patented novel colour-switching technologies and tuneable lasers incorporating heliconical nematic phases. More recent research has involved the inclusion of bulky heteroatoms such as sulfur in the molecular structure and the synthesis of longer liquid crystal oligomers.

Textures of the twist-bend nematic phase obtained using polarised light microscopy. - Chiral twist-bend nematic phase
-
The NTB phase may be considered the generalised case of the chiral nematic phase, N*, the director of which is orthogonal to the helical axis. The N* phase is exhibited by chiral molecules and the molecular chirality provides the driving force for the formation of the helical structure. The pitch length of the N* phase is typically several hundred nanometres, some two orders of magnitude larger than that found in the NTB phase (~10 nm).

Polarised light microscope images showing the textures of the chiral twist-bend nematic phase.Our work addresses the intriguing question as to how the NTB phase having spontaneous structural chirality will respond at a microscopic level to the presence of intrinsic molecular chirality. Theory predicts the double degeneracy of helical twist sense in the NTB phase will be removed by the introduction of molecular chirality - as shown in our cartoon! - and such chiral doping will increase the stability of the resulting phase. We have recently reported the first example of a chiral twist-bend nematic phase (termed N*TB) in a molecule with a single chiral centre, and directly compared it to its racemic analogue. The N*TB-N* transition temperatures are higher for the chiral dimers than seen for the racemic counterparts and thus these preliminary experimental studies are in accord with theoretical predictions. The chiral and 'conventional' twist-bend nematic phases also exhibit strikingly different optical textures when viewed down the polarised optical microscope, and our future work aims to establish the origins of these microscale differences.

Work on the chiral twist-bend nematic phase featured on the back cover of Chemistry: A European Journal.
- Twist-bend smectic phases
-
Along with the twist-bend nematic phase, theory predicted the existence of short pitch heliconical smectic phases composed of achiral molecules. Recent studies have shown that this lamellar analogue of the NTB phase indeed exists, and we termed this the SmCTB phase. For some molecular systems, the SmCTB phase has a simple heliconical arrangement with the molecules changing azimuthal direction of tilt by a constant angle when going from layer to layer, i.e., forming a clock-like structure, and the helical pitch is as short as that found for the NTB phase, about 3-4 smectic layers. For other systems, however, the SmCTB structure is more complex, with an additional longer helix with the pitch of the order of tens of smectic layers superimposed on a short helix.
Such a system has the exciting potential to serve as a model for more complex materials in which a hierarchical structure is propagated from the molecular to the nano- or micro-scale. For example, the chirality of biological molecules such as nucleic acids and amino acids is, in part, responsible for the helical structure of DNA and proteins, which in turn leads to the lack of mirror symmetry of macro-bio-objects.
If this secondary helix unwinds and its pitch becomes comparable to the optical wavelength, this will lead to the well-known phenomenon of the selective reflection of light with an energy band gap in the visible range.. This effect, although common for chiral liquid crystal phases, we have very observed for the first time using achiral mesogens.
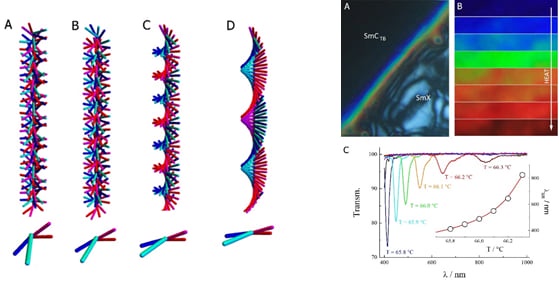
Sketch of the twist-bend smectic C phase showing changes in structure with temperature. (left) Selective reflection of light across the visible light range in one of our twist-bend smectic materials. (right) - Hydrogen-bonded liquid crystals
-
While most of the so-called conventional liquid crystals comprise molecular compounds assembled via weak interactions, hydrogen-bonding represents an extremely versatile strategy to form new supramolecular entities with mesomorphic properties. In our group, we reported in 2015 the first example of a supramolecular trimer to exhibit the NTB phase, assembled by hydrogen-bonding between two similar benzoic acids that leads to a helicoidal superstructure. Later in 2018, we extended our strategy by assembling unlike molecules, widening the possibility to form new functional NTB materials beyond covalent chemistry. One exciting challenge for us is control of the resulting geometries (and therefore properties) of new supramolecular dimers, oligomers and polymers by promoting specific interactions in new hydrogen-bonded systems in a rational manner by combining computational modelling techniques and advanced FT-IR and dielectric spectroscopic methods.
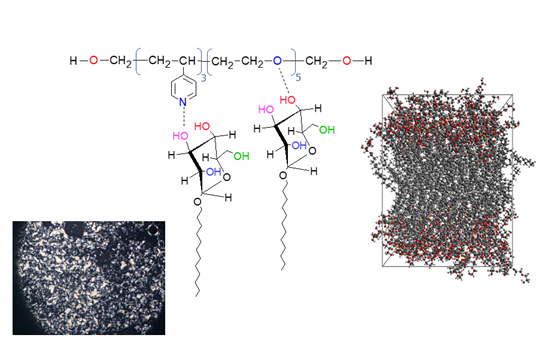
Smectic phases formed by polymers complexed with glysosides obtained from natural sources, via hydrogen-bonding.
Our work on hydrogen-bonded dimers featured in Materials Advances. - Liquid crystals for energy applications
-
As part of Dr Martinez-Felipe's research interests, and following an extensive legacy in our Department on ionically conducting materials, we have developed and characterised a series of new liquid crystalline materials with the potential to be used as electrolytes for energy conversion and storage. This has included side-chain polymers containing polar groups that promote ionic motions through ordered microstructures assisted by smectic A phases, and ionic liquid crystals exhibiting smectic T phases and very high conductivity under anhydrous conditions. We are actively exploring how the inclusion of azobenzenes, capable of undergoing reversible cis-to-trans isomerisations, can lead to a broad range of new functional materials for solar cells and photovoltaic applications.
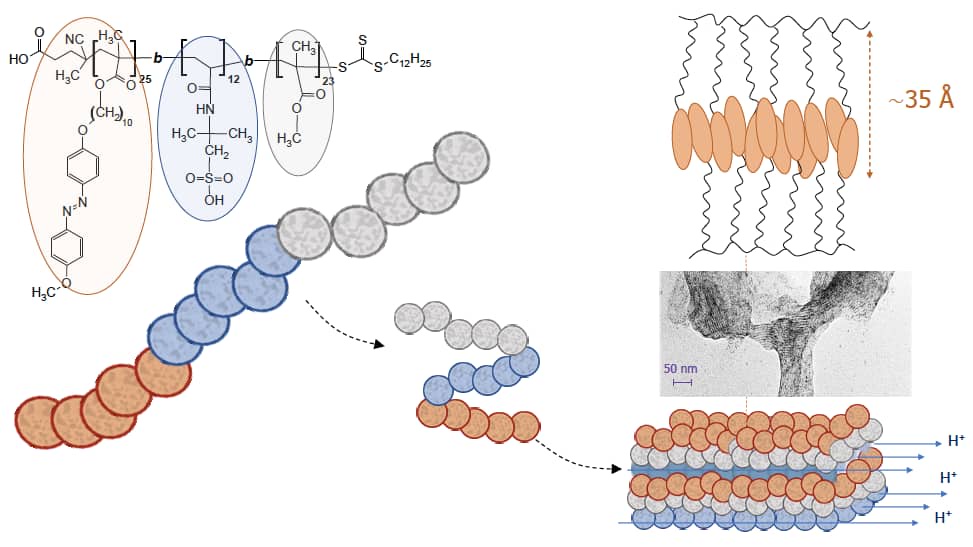
Light-responsive block copolymers containing polar groups that promote conductivity through smectic domains. - The ferroelectric nematic phase
-
In the conventional uniaxial N phase, the director is a unit vector having inversion symmetry (ie. n = -n), and so the phase is described as non-polar. Almost 100 years ago, an alternative model of the nematic phase was proposed, in which the molecular dipole moments, if strong enough, would also become ordered, such that n ≠ -n. It was predicted that in such a phase, the ferroelectric nematic (or 'ferronematic') phase NF, the polarity of the phase may induce a spontaneous chirality through steric and electrostatic interactions between molecules. This phase was only very recently experimentally discovered and at present is incredibly rare, appearing thus far in only a handful of materials. Not only are these materials of great fundamental interest - investigations into their as-yet unknown physical properties have only just begun to scratch the surface - the combination of ferroelectricity with nematic-like fluidity and ease of processing would have enormous potential for applications. Our current research focusses on finding new examples of materials exhibiting the NF phase and optimising the temperature at which this phase occurs through structural modifications.
Selected Publications
- Pociecha, D., Szydlowska, J., Kwiatkowska, K., Judoka, M., Spiess, J., Storey, J.M.D., Imrie, C.T., Walker, R. and Gorecka, E. Twist-grain boundary phases in the proper ferroelectric liquid crystals realm. Advanced Science. (2025)
- Cruickshank, E., Walker, R., Majewska, M., Gorecka, E., Pociecha, D., Storey, J.M.D., and Imrie, C.T. The influence of methyl groups on the formation of the ferroelectric nematic phase. ACS Omega. 10 (22), 23609-23619. (2025)
- Alshammari, A., Zattarin, A., Pearson, A., Cruickshank, E., Majewska, M., Pociecha, D., Storey, J.M.D., Gorecka, E., Imrie, C.T. and Walker, R. Heliconical nematic and smectic phases: the synthesis and characterisation of the CB4O.m and CB8O.m series. Liquid Crystals. 51(13-14), 2300-2312. (2024)
- Cruickshank, E., Rybak, P., Majewska, M., Ramsey, S., Wang, C., Zhu, C., Walker, R., Storey, J.M.D., Imrie, C.T., Gorecka, E. and Pociecha, D. To Be or Not To Be Polar: The Ferroelectric and Antiferroelectric Nematic Phases. ACS Omega. 8, 39, 36562-36568. (2023)
- Szydlowska, J., Majewski, P.W., Cepic, M., Vaupotic, N., Rybak, P., Imrie, C.T., Walker, R., Cruickshank, E., Storey, J.M.D., Pociecha, D. and Gorecka, E. Ferroelectric nematic-isotropic critical end point. Physical Review Letters. 130(21), 216802 (2023)
- Cruickshank, E., Pearson, A., Brown, S., Storey, J.M.D., Imrie, C.T. and Walker, R. The Ferroelectric Nematic Phase: On the role of lateral alkyloxy chains. Liquid Crystals. 50(11-12), 1960-1967. (2023)
- Meyer, C., Davidson, P., Luckhurst, G.R., Dokli, I., Knezevic, A., Lesac, A., Paterson, D.A., Walker, R., Storey, J.M.D., Imrie, C.T. and Dozov, I. Temperature Dependence of the Electroclinic Effect in the Twist-Bend Nematic Phase. Crystals, 13(3), 465. (2023)
- Walker, R., Pociecha, D., Salamonczyk, M., Storey, J.M.D., Gorecka, E. and Imrie, C.T. Intrisically Chiral Twist-Bend Nematogens: Interplay of Molecular and Structural Chirality in the NTB phase. ChemPhysChem. DOI: 10.1002/cphc.202200807. (2022) (FEATURED ARTICLE: front cover, cover profile)
- Imrie, C.T., Walker, R., Storey, J.M.D., Gorecka, E. and Pociecha, D. Liquid Crystal Dimers and Smectic Phases: From the Intercalated to the Twist-Bend. Crystals. 12, 1245. (2022) (Invited Review, Special Issue: State-of-the-Art Liquid Crystals Research in UK)
- Pociecha, D., Walker, R., Cruickshank, E., Szydlowska, J., Rybak, P., Makal, A., Matraszek, J., Wolska, J.M., Storey, J.M.D., Imrie, C.T. and Gorecka, E. Intrinsically chiral ferronematic liquid crystals. Journal of Molecular Liquids. 361, 119532. (2022)
- Alshammari, A., Pociecha, D., Walker, R., Storey, J.M.D., Gorecka, E. and Imrie, C.T. New patterns of twist-bend liquid crystal phase behaviour: the synthesis and characterisation of the 1-(4-cyanobiphenyl-4’-yl)-10-(4-alkylaniline-benzylidene-4’-oxy)decanes (CB10O.m). Soft Matter. 18, 4679-4688. (2022)
- Walker, R., Pociecha, D., Faidutti, C., Perkovic, E., Storey, J.M.D., Gorecka, E. and C.T. Imrie. Remarkable stabilisation of the intercalated smectic phases of nonsymmetric dimers by tert-butyl groups. Liquid Crystals. 49, 969-981. (2022)
- Pociecha, D., Vaupotic, N., Majewska, M., Cruickshank, E., Walker, R., Storey, J.M.D., Imrie, C.T., Wang, C. and Gorecka, E. Photonic Bandgap in Achiral Liquid Crystals – A Twist on a Twist. Advanced Materials. 33(39), 2103288. (2021)
- M. Salamonczyk, N. Vaupotic, D. Pociecha, R. Walker, J.M.D. Storey, C.T. Imrie, C. Wang, C. Zhu, E. Gorecka. Multi-level chirality in liquid crystals formed by achiral molecules. Nat Commun., 10, 1922 (2019).
- D. Pociecha, C.A. Crawford, D.A. Paterson, J.M.D. Storey, C.T. Imrie, N. Vaupotic, E. Gorecka. Critical behavior of the optical birefringence at the nematic to twist-bend nematic phase transition. Phys. Rev. E., 98, 052706 (2018).
- R. Walker, D. Pociecha, J. P. Abberley, A. Martinez-Felipe, D. A. Paterson, E. Forsyth, G. B. Lawrence, P. A. Henderson, J. M. D. Storey, E. Gorecka and C. T. Imrie. Spontaneous chirality through mixing achiral components: a twist-bend nematic phase driven by hydrogen-bonding between unlike components. Chem. Commun., 54, 3383-3386. (2018)
- J.P. Abberley, R. Killah, R. Walker, J.M.D. Storey, C.T. Imrie, M. Salamonczyk, C. Zhu, D. Pociecha, E. Gorecka. Heliconical smectic phases formed by achiral molecules. Nat. Commun., 9, 228 (2018).
- D. A. Paterson, J. Xiang, G. Singh, R. Walker, D.M. Agra-Koojiman, A. Martinez-Felipe, M. Gao, J.M.D. Storey, S. Kumar, O.D. Lavrentovitch and C.T. Imrie. Reversible Isothermal Twist-Bend Nematic-Nematic Phase Transition Driven by the Photoisomerization of an Azobenzene-Based Nonsymmetric Liquid Crystal Dimer. J. Am. Chem. Soc., 138, 5283-5289 (2016).
- S.M. Jansze, A. Martinez-Felipe, J.M.D. Storey, A.T.M. Marcelis and C.T. Imrie. A Twist-Bend Nematic Phase Driven by Hydrogen Bonding. Angew. Chem. Int. Ed., 54, 643-646 (2015).
