| Projects undertaken by ACE have contributed to the development of many different research tools for both researchers and users of research. In collaboration with colleagues at other Universities we have developed tools to help trialists match their trial design decisions to the needs of the intended users of the trial results. |
 |
- Study Design and Sample Size Calculator
-
Sample Size calculator for cluster randomised trials
Version 2 of the sample size calculator is in development.
Please click here for a beta version. See also this document.The instruction manual for this program can be downloaded as a pdf file at the link below.
A further description of the calculator can be found in Campbell MK, Thomson S, Ramsay CR, MacLennan GS, Grimshaw JM. Sample size calculator for cluster randomised trials. Comput Biol Med 2004;34:113-125.
If you have any queries about the calculator, please contact Jemma Hudson (j.hudson@abdn.ac.uk).
PRECIS-2 Tool
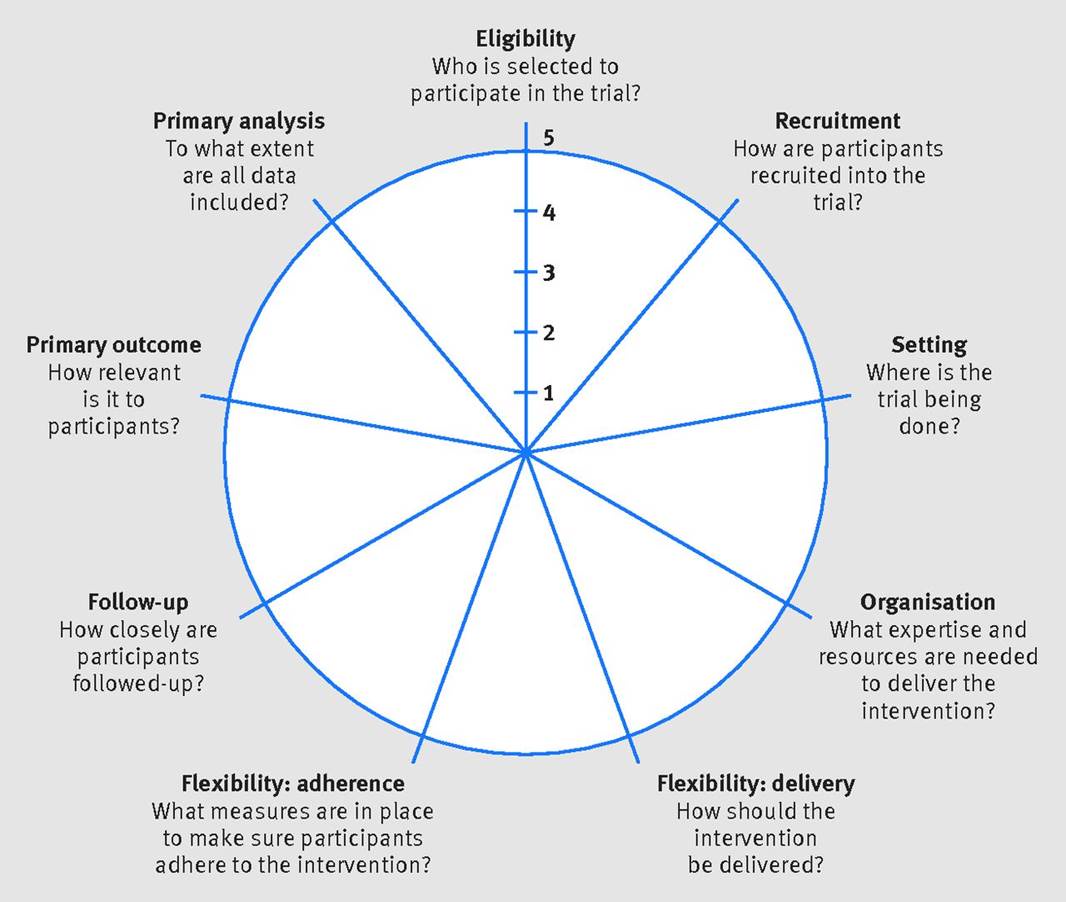
In collaboration with colleagues at other Universities we have developed PRECIS-2, which is a tool to help trialists match their trial design decisions to the needs of the intended users of the trial results. PRECIS-2 has nine domains—eligibility criteria, recruitment, setting, organisation, flexibility (delivery), flexibility (adherence), follow-up, primary outcome, and primary analysis—scored from 1 (very explanatory design approach) to 5 (very pragmatic design approach) to facilitate domain discussion and consensus.
A website to support the use of the tool is available at www.precis-2.org
A description of PRECIS-2 can be found in Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ 2015;350:h2147 (http://www.bmj.com/content/350/bmj.h2147)
If you have any queries about the calculator, please contact Shaun Treweek (streweek@mac.com).
Database of intra-correlation coefficients (ICCs)
A downloadable excel spreadsheet contains a list of intra-correlation coefficient calculated from a number of different interventions and settings. Also downloadable, is a list of references for the data contained in the spreadsheet.
- Implementation studies: Spreadsheet
- Implementation studies: References
- Surgical studies: Spreadsheet
Contamination in randomised trials
A flowchart was developed by the HTA Contamination in trials project to aid the decision on when a cluster randomised controlled trial might be preferred to a patient randomised controlled trial when contamination of controls are suspected.
INCLUDE Ethnicity Framework
Trial teams need to do everything possible to make their trial relevant to the people for whom the results are intended to apply (often patients) and those expected to apply them (often healthcare professionals).
The INCLUDE Ethnicity Framework aims to help trial teams think carefully about which ethnic groups should be included in their trial for its results to be widely applicable, and what challenges there may be to making this possible. Having identified potential challenges, the trial team can then consider ways to reduce those challenges. For this to work best, the Framework needs to be used at the trial design stage before funding is in place.
Click here for more information.
INCLUDE Socioeconomic Disadvantage Framework
This framework has been designed to aid researchers, who are designing clinical trials, to consider barriers to including patients from socioeconomically disadvantaged backgrounds in their trial. The framework can also help researchers to develop strategies to attempt to address such barriers in order to improve the design and conduct of clinical research. Although this framework was developed with UK-based clinical trials in mind, aspects may also be relevant to different types of research and research conducted in populations outside of the UK. Whilst this framework focuses on socioeconomic disadvantage, the list of underserved groups in clinical research is extensive and researchers need to be aware of this when identifying barriers to research and developing to strategies to address barriers.
Click here for more information.
Multilevel factorial cluster randomised controlled trials
Complex randomised controlled trials are required to evaluate complex interventions.
Multilevel factorial cluster randomised controlled trials (C-RCT), also known as split-plot designs, address research questions that are multilevel (interventions aimed at different levels in a structure such as doctors and patients) and have the potential to explore interactions between interventions (whether or not giving two interventions together works any better than giving them separately.
To learn more about this type of design and its statistical implications, please consult Beatriz Goulao's presentation here.
We have published a set of guidelines to improve the reporting of multilevel factorial cluster randomised controlled trials, which you can see here.
A statistical simulation tutorial to aid the sample size calculation for multilevel factorial cluster randomised controlled trials will be available soon.
Find out about Beatriz's Goulao's PhD project here.

- Study Conduct
-
Governance
The DAMOCLES study formulated a list of considerations that would be valuable for a Data Monitoring Committee (DMC) to address at the start of a trial which were developed into a charter.
- Charter for DMCs: template (Word document)
- Worked example of the DAMOCLES charter (Word document)
- Outcome Measures
-
Beliefs about surgery questionnaire
The REFLUX Study developed and validated a questionnaire for measuring patients Beliefs about surgery.
- Patient and public involvement in numerical aspects of research
-
As part of this programme of research, we have developed tools to support communication of numerical or statistical aspects of trials with patients and the public.
As part of the PoINT project, we developed a guide for numerical aspects developed by Beatriz Goulao and Richard Caie and funded by the Wellcome Trust ISSF fund at the University of Aberdeen: download here, and find out more about the results of the project here.
As part of the INITIAL project, we developed a blog with lay summaries of statistical aspects of trials to support patient and public involvement in statistics in trials.
- Randomisation Service
-
ACE provide validated and easy to use online/IVR (telephone) software for randomising patients into clinical trials.
Reasons to choose ACE for your service:
- Highly experienced staff and reliable online/telephone service.
- Can integrate and export data dynamically to other 3rd party systems.
- Suitable for a wide variety of trial types, customised to your trial.
- Advice on system design and implementations.
- Ongoing export support via online support tool.
Simple randomisation, block randomisation, stratified block randomisation and minimisation are offered as standard. Novel randomisation can be implemented on request.
Telephone randomisation service
ACE offer a 24-hour, seven-day automated telephone randomisation service, telephone calls are made to the service using a Freephone number, which is connected to 16 telephone lines. For international calls, there is a standard UK telephone number.
Web-based randomisation service
ACE offer a secure and robust web-based randomisation system. It is accessible anytime, anywhere and via any device that has a modern web browser and is connected to the Internet.
Standard features
A customisable role-based user access control system, email notifications are built in. The randomisation system generates confirmation emails that can be blinded or unblinded to treatment allocation.
Standard reports summarising randomisation and all data-entry activity, custom reports implemented on request.
All transactions are logged, the trial's audit trail and the list of randomisations can be downloaded and analysed at any time by authorised users
Randomisation simulation data is generated before the study begins, to check balance across stratification factors. The system can be programmed to perform validation checks, such as checking for duplicate Participant Identification Numbers and preventing randomisation from proceeding in such cases.
Drug supply chain management
The randomisation service can also handle drug supply chain. Active and placebo unique treatment pack numbers, batch numbers and expiry dates can be programmed into the system, with the system recording packs delivered to specific site/pharmacies and only allocating medication packs that are available at that site. Notifications can be set up to alert site/pharmacies of low drug supply, and this functionality is configurable as required. For example, notify site/pharmacy when the stock level reaches x amount of packs.
Unblinding
We can offer you an emergency online/telephone code break (unblinding) service which is also available 24 hours a day. Investigators break the blind by simply providing the unique randomisation number, or other identifier used for your trial medication or intervention. Investigators are immediately notified of the unblinded treatment allocation. Trial teams can choose to be notified by email of every new code break.
ePRO
Invitations are sent by email, links to complete the forms online are included in the emails. Invites and reminders can be sent automatically via our built-in configurable communication manager.
Implementation and cost
The cost of the service depends on how long the service is required and the sample size. The randomisation request form can be found here.
If you require further information relating to planning a new randomisation service or If you would like a demonstration on either the telephone or web-based randomisation service, please contact the Senior IT Development Manager: Mark Forrest
- Review and Dissemination
-
Projects have contributed to guidance documents for reviewing and disseminating results of research.
- Producing information about health and health care interventions: a practical guide (PDF)
- How to identify and assess evidence from diagnostic studies of imaging: an introductory guide (PDF)
- “Sharing trial results directly with trial participants and other stakeholders after the SARS-CoV-2 pandemic hit the UK - experience from the ActWELL trial” https://trialsjournal.biomedcentral.com/articles/10.1186/s13063-021-05340-3
