
Publication
Our research team have published a systematic review and meta-analysis in PLOS ONE, taking a look at NIPT implementation worldwide.
Funded by the Medical Research Scotland PhD Studentships Programme
Researchers at the University of Aberdeen, in collaboration with Public Health Scotland (PHS), are investigating the impact of introducing a new screening test – non-invasive prenatal testing (NIPT) - into the Scottish antenatal screening programme for Down’s syndrome.
They will try to understand whether introducing NIPT has changed:
This project builds on previous work by the Congenital Conditions and Rare Diseases Registration Information Service for Scotland (CARDRISS) team in Public Health Scotland, specifically the creation of the Scottish linked congenital conditions dataset (SLiCCD). This important resource captures data on all pregnancies and births diagnosed with a congenital condition in Scotland since 2000. Really, this is what makes the analysis in our project possible – the ability to look at a population level at the impact of changes to the Scottish screening programme for specific congenital conditions."
- Dr Rute Vieira, Principal Supervisor of the Doctoral project
This doctoral project 
The study will analyse population-wide healthcare, congenital condition register, and screening and genetic datasets to evaluate the real-world impact of introducing NIPT into the screening programme for Down's syndrome in Scotland. This includes assessing the impact of NIPT on screening and continuation of pregnancy decisions made by women, as well as how long babies with Down’s syndrome live. The project will also examine any changes in medical care received by babies with Down's syndrome.

Every child with Down’s syndrome is unique, following the same developmental path as all children. Most individuals with Down’s syndrome will have some form of learning impairment, and some will have more complex needs, although everyone is different.
Many people with Down’s syndrome lead happy and fulfilled lives, living as independently as possible and pursuing activities and occupations into their 60s, 70s and beyond. More information and insight into the lives of people with Down’s syndrome can be found through the following websites:
Non-invasive prenatal testing was introduced to the Scottish antenatal screening programme in September 2020.
Routine screening was available for Down’s syndrome only and was offered to all women with a singleton pregnancy. Women that opted for screening tests were given a chance score for the baby having Down’s syndrome. This score was based on a combination of maternal blood samples and ultrasound measurements, depending on the gestation of pregnancy. Down's syndrome screening for twin pregnancies was only offered in the first trimester.
If a pregnancy was given a higher chance of Down’s syndrome (i.e. >= 1 in 150), the mother was offered an invasive test to confirm the condition. These diagnostic tests (amniocentesis or chorionic villus sampling) have, however, a small risk of miscarriage.
At each stage, women could decide not to have any further testing.
This screening pathway for Down’s syndrome is represented in Figure 1.
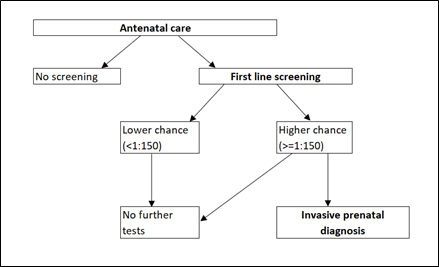
Figure 1: Flow diagram to represent the screening options for Down's syndrome for a singleton pregnancy before September 2020.
All pregnant women booking for antenatal care are now offered screening for Down’s, Edwards' and Patau’s syndromes if they are in their first three months of pregnancy. Women that opt for screening tests, are given a chance score for the baby having either Down’s syndrome or, separately, either Edwards’ or Patau’s syndrome, based on the same testing as before (now referred to as first line screening). Screening has also been extended to twin pregnancies in the first and second trimester for Down’s syndrome.
If the chance of the baby having a specific condition is higher, women are offered another screening test called non-invasive prenatal test (NIPT), for which women only need to give a blood sample and will be told whether there is a high or low chance that the baby has the condition.
NIPT is a more accurate screening test for these three conditions, and it will hopefully reduce the need for pregnant women to undergo invasive tests. However, women can still choose to do an invasive test instead, or no further screening tests (Figure 2).
If the NIPT result is positive, women are then offered invasive testing, or no further testing.
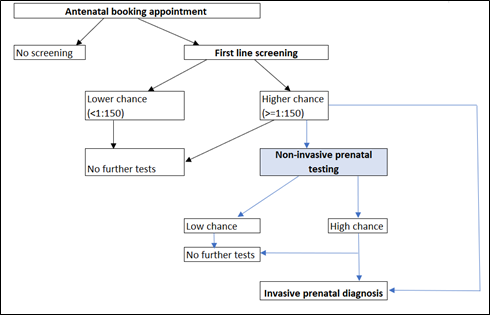
Figure 2: Screening options available for singleton pregnancies for Down's syndrome following the September 2020 implementation of NIPT. First line screening test refers to the combined or quadruple screening available before NIPT was introduced.
When used as a screening test, NIPT has been found to have over 99% accuracy for Down’s syndrome (Taylor-Philips et al., 2016), meaning it is much more accurate than the first line screening tests. However, this is still a screening test, and there is a very small chance of being incorrect. Therefore, NIPT is not enough to diagnose a baby with Down’s syndrome. To get a definitive answer for whether a baby has Down’s syndrome before birth, invasive testing such as amniocentesis or chorionic villus sampling, must still be used.
For further information on NIPT please visit the NHS inform website.
The implementation and impact of non-invasive prenatal testing (NIPT) for Down’s syndrome into antenatal screening programmes: A systematic review and meta-analysis
Sebire E, Rodrigo CH, Bhattacharya S, Black M, Wood R, Vieira R (2024) The implementation and impact of non-invasive prenatal testing (NIPT) for Down’s syndrome into antenatal screening programmes: A systematic review and meta-analysis. PLoS ONE 19(5): e0298643. https://doi.org/10.1371/journal.pone.0298643
Pregnancy Screening for Down’s Syndrome, Edwards’ Syndrome, and Patau’s Syndrome in Scotland
Public Health Scotland Official Statistics in Development: Pregnancy Screening for Down’s Syndrome, Edwards’ Syndrome, and Patau’s Syndrome in Scotland. https://publichealthscotland.scot/publications/pregnancy-screening-for-down-s-syndrome-edwards-syndrome-and-patau-s-syndrome-in-scotland/pregnancy-screening-for-down-s-syndrome-edwards-syndrome-and-patau-s-syndrome-in-scotland/
To understand where NIPT has been offered by national health systems around the world, and how this has been achieved.
To check how well the different datasets from Public Health Scotland correctly identify babies with Down’s syndrome in Scotland.
To understand how NIPT influences the decisions that women make concerning screening and continuation of pregnancy, as well as how long babies with Down’s syndrome live and medical care of babies with Down’s syndrome.
To examine how future changes in the uptake of antenatal screening may impact babies with Down’s syndrome by using a decision analysis.

PhD student at the University of Aberdeen.

Senior Lecturer in Medical Statistics at the University of Aberdeen and consultant for Public Health Scotland.

Senior Lecturer in Public Health based at the School of Medicine, Medical Sciences and Nutrition at the University of Aberdeen.

Consultant in Public Health Medicine at Public Health Scotland.
For more information, please contact Dr Rute Vieira.
Very sadly, Dr Sohinee Bhattacharya passed away in the summer of 2023. She was a treasured member of our team and, as secondary supervisor, was pivotal in the conception and progress of the project for the last two years. Sohinee brought her much respected clinical and academic expertise to this project, and her contributions and valuable insights to this work will be long remembered.