
Personal Chair
- About
-
- Email Address
- h.deng@abdn.ac.uk
- Telephone Number
- +44 (0)1224 272953
- Office Address
- School/Department
- School of Natural and Computing Sciences
Biography
08/2022 - Personal Chair, University of Aberdeen, UK
08/2018 - 07/2022 Reader, University of Aberdeen, UK
08/2014 - 07/2018 Senior Lecturer, University of Aberdeen, UK
10/2008 - 07/2014 Lecturer, University of Aberdeen, UK
06/2002 - 09/2008 Postdoctoral fellow, University of St Andrews, UK
1999 - 2002 Ph.D. University of Wales Swansea, UK
Memberships and Affiliations
- Internal Memberships
-
Director of Research
PGR coordinator
CM2514 course coordinator
- External Memberships
-
BBSRC pool of expert
MRC and BBSRC panel members
UKRI FLF peer review colleague
External reviewer for a series of peer-reviewed journals within my area of interest, including Nature, Nat. Chen. ACIE, Sci. Adv. Chem. Sci., Chem Comm., ACS Chem. Biol., ChemBioChem, Marine Biotechnology, Org & Biomol. Chem, FEMS Microbiology Ecology, Chemistry today, Marine Drugs, Fish and Shellfish Immunology, and Virulence.
A reviewer of research councils, BBSRC (UK) and FCT (Fundação para a Ciência e a Tecnologia in the field of Chemistry and Biochemistry, 2010-2011), Fonds de recherche du Québec - Nature et technologies, Québec, Canada (2015-2016) and charities, Leverhulme Trust (UK).
- Research
-
Research Overview
My research carries on the great tradition of UK natural product biosynthesis activity. We have all the required skills to succeed in such an enterprise, involving discovery of new families of specialised metabolites, bacterial genomics, chemical synthesis and the associated molecular biology to manipulate genomes to enzymology and the reconstruction of biochemistry. All of this is focussed on understanding the molecular basis and mechanism of natural products assembly.
Recent contributions involve the understanding of a group of unsaturated amino acids called dehydroamino acids (dhAAs). dhAAs are key components in many peptidyl therapeutics and versatile building blocks in peptide chemistry where organic chemists generate peptidyl derivatives through Michael additions, cross couplings and cycloaddition. They are used as bio-orthogonal handles for later stage modification of biomolecules. However, accessing dhAAs in structurally complex molecules presents a synthetic challenge, often resulting in poor atomic economy.
Among approximately 40 dhAAs found in the natural product inventory, only two, dehydroalanine (Dha) and (Z)-dehydrobutyrine (ZDhb), are well-studied. In this context our lab has elucidated the formation of several understudied dhAAs in newly discovered bacterial peptide-related metabolites, such as pyrrolizidine alkaloids (PAs) (Angew Chem. Int. Ed. 2015, 54, 12697, Nat. Comm. 2022, 13, 62), short dehydrated non-ribosomal peptides (Angew Chemie. Int. Ed. 2021, 60, 3229) and a new family of ribosomally synthesized and posttranslationally modified peptides (RiPPs) (Nat. Comm. 2022, 13, 5044) as shown below. Apart from this, we have also accumulated an outstanding body of work from bioactive natural product discovery to new enzymology as evidenced in our publication profile.
The discovery we made offers an alternative to rational engineering of pathways to generate bioactive peptides. As such we have obtained grants from various funding bodies (i.e. UKRI, Leverhulm Trust, Royal Society, Royal Society of Edinburgh, IBIOIC, EC) to further investigate potential applications of novel enzymes identified in these pathways towards the development of pharmaceuticals.

Research Areas

Biological and Environmental Sciences

Chemistry
Research Specialisms
- Industrial Biotechnology
- Biochemistry
- Organic Chemistry
- Applied Chemistry
Our research specialisms are based on the Higher Education Classification of Subjects (HECoS) which is HESA open data, published under the Creative Commons Attribution 4.0 International licence.
Past Research
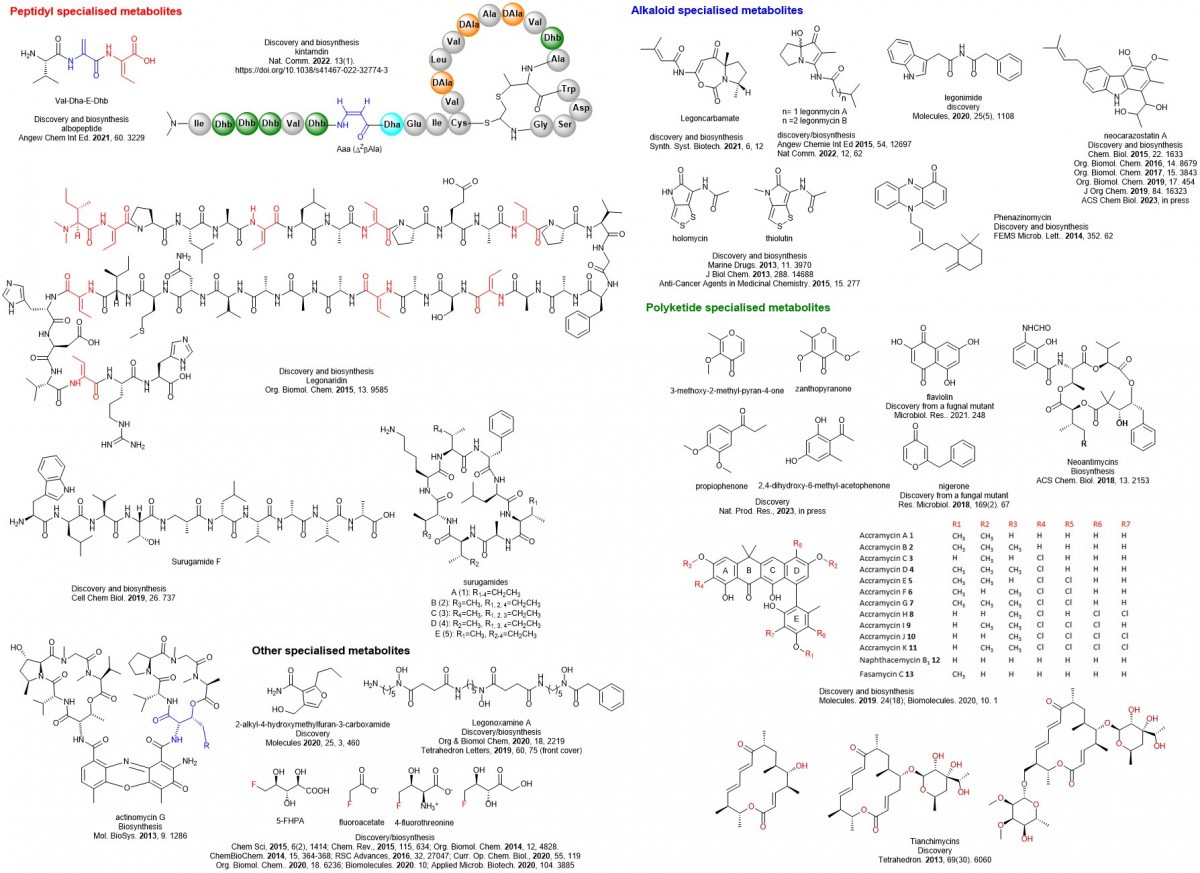
My team has accumulated a good body of work on bioactive natural product discovery (from polyketides and peptides to alkaloids) and their biosynthesis, and new enzyme investigation as shown below. Collectively it is a combination of both its range and illustration and these new organic molecules and new enzymes my team has discovered will find applications for the development of pharmaceuticals and agrochemicals or new ways to biosciences.
Collaborations
Professor Mathew Jenner, Department of Chemistry, University of Warwick, UK
Professor Steven Cobb, Department of Chemistry, University of Durham, UK
Dr Jioji Tabudravu, School of Natural Sciences, University of Central Lancashire, UK
Dr David Clarke, EastChem, School of Chemistry, University of Edinburgh, Edinburgh, EH9 3FJ, UK
Professor Bruce Milne, Department of Physics, University of Coimbra, Rua Larga, 3004-516, Coimbra, Portugal
Professor Yi Yu, School of Pharmaceutical Sciences, Wuhan University, Wuhan 430071, China
Professor Kwaku Kyeremeh, Department of Chemistry, University of Ghana, Ghana
NCIMB Ltd and Ingenza Ltd
Funding and Grants
We gratefully thank the funding bodies below for financial supports of our research
MRC, BBSRC, Leverhulm Trust, EC, IBIOIC, The Royal Society and The Royal Society of Edinburgh.

- Teaching
-
Teaching Responsibilities
CM4518 Biological origin of natural products
CM1020 Chemistry for Biosciences 1
CM1512 Chemistry for Biosciences 2
CM3534 Organic and Biological Chemistry
- Publications
-
Page 10 of 11 Results 91 to 100 of 109
Identification and heterologous expression of the biosynthetic gene cluster for holomycin produced by Streptomyces clavuligerus
Process Biochemistry, vol. 46, no. 3, pp. 811-816Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1016/j.procbio.2010.11.024
An enzymatic route to 5-deoxy-5-[18F]fluoro-D-ribose, a [18F]-fluorinated sugar for PET imaging
Chemical Communications, vol. 46, no. 1, pp. 139-141Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1039/b919364b
Mechanistic insights into water activation in SAM hydroxide adenosyltransferase (duf-62)
ChemBioChem, vol. 10, no. 15, pp. 2455-2459Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1002/cbic.200900369
In vitro reconstituted biotransformation of 4-fluorothreonine from fluoride ion: Application of the fluorinase
Chemistry & Biology, vol. 15, no. 12, pp. 1268-1276Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1016/j.chembiol.2008.10.012
The fluorinase, the chlorinase and the duf-62 enzymes
Current Opinion in Chemical Biology, vol. 12, no. 5, pp. 582-592Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1016/j.cbpa.2008.06.036
S-Adenosyl-L-methionine:hydroxide adenosyltransferase: a SAM enzyme
Angewandte Chemie International Edition, vol. 47, no. 29, pp. 5357-5361Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1002/anie.200800794
Fluorine and Health: Biological Fluorination in Streptomyces cattleya: The Fluorinase
Elsevier. 1st edition, pp. 761-777, 17 pagesChapters in Books, Reports and Conference Proceedings: Chapters- [ONLINE] DOI: https://doi.org/10.1016/B978-0-444-53086-8.00018-7
The identification of (3R, 4S)-5-fluoro-5-deoxy-D-ribulose-1-phosphate as an intermediate in fluorometabolite biosynthesis in Streptomyces cattleya
Bioorganic Chemistry, vol. 35, no. 5, pp. 375-385Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1016/j.bioorg.2007.04.001
The fluorinase from Streptomyces cattleya is also a chlorinase
Angewandte Chemie International Edition, vol. 45, no. 5, pp. 759-762Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1002/anie.200503582
Fluorinase mediated C--18 F bond formation, an enzymatic tool for PET labelling
Chemical Communications, no. 6, pp. 652-654Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1039/B516861A
