
Personal Chair
- About
-
- Email Address
- peter.mccaffery@abdn.ac.uk
- Telephone Number
- +44 (0)1224 437362
- Office Address
Institute of Medical Sciences
Room 4:33
Foresterhill
Aberdeen AB25 2ZD- School/Department
- School of Medicine, Medical Sciences and Nutrition
Biography
I graduated in Biochemistry at Victoria University, New Zealand and obtained a Ph.D. in Pathology at Otago University, New Zealand in 1987. After post-doctoral research at Harvard Medical School I became Instructor and then Assistant Professor in the Department of Psychiatry, Harvard Medical School, where I first developed my interest in retinoic acid in the developing central nervous system. After working at the University of Massachusetts Medical School, Worcester, MA as Associate Professor in Cell Biology, I moved to the University of Aberdeen in 2006. There I was co-director of the Institute of Medical Sciences until 2015. My research into retinoic acid continues with a focus on its function in the hypothalamus as well as its potential to protect in neuropsychiatric and neurodegenerative disorders, the latter including Alzheimer’s and motor neuron disease. My publications can be viewed on google scholar with an h-index of 55.
- Research
-
Research Overview
CNS Vitamin A Research Laboratory
We all grow up with the idea that vitamins are good for us and we will become very sick if deficient. Very little is known though about the need of vitamin A for the brain. Our laboratory has demonstrated that vitamin A is essential for the brain and necessary for some of its primary functions and how it controls the rest of the body. This research uses in-vitro cell lines, studies the brains of mice and rats as well as translating this research to humans. Working in the laboratory (but sometime in the evening enjoying an Indian meal) are, from left to right Azita Kouchmeshky (PhD student), Peter McCaffery (PI), Cristina Lagido (former Technician), Elizabeth Hay (former honours student now Resarch Fellow at the Univ. Aberdeen), Reem Bu Saeed (former PhD student now Assistant Professor KSAU-National Guard, Jeddah, Kingdom of Saudi Arabia), Thabat Khatib (former PhD student now Resarch Fellow at Hebrew Univ. Jerusalem) and, thanks to the magic of photoshop, Jason Clark (PhD student) while a future neuroscientist is in the foreground (Reem's daughter, Rafeef).

To HEAR some our research go to Naked Scientist "Science in Scotland" podcast.
To READ some more of our views on nutrients and vitamins head to "The Conversation".
To VIEW AN ANIMATION of how retinoic works click here for a movie (or here for an annotated version, courtesy C. McCaffery)
To YOUTUBE view a clip of one of our projects head here
To view the COMPANY that arose from this work and led by Andy Whiting go to "Nevrargenics Ltd"
What is vitamin A and what does it do?
Vitamins are compounds in food that, in just small amounts, are required for growth and maintaining good health. Vitamin A is a lipid, often in the chemical form retinol, required for the function of the brain, as well as many other organs. It can be converted to an acidic form called retinoic acid which controls gene expression (see below). For many of Vitamin A’s functions its conversion to retinoic acid is essential. Cells that require retinoic acid capture vitamin A from the circulation and contain the enzymes that convert it to retinoic acid.
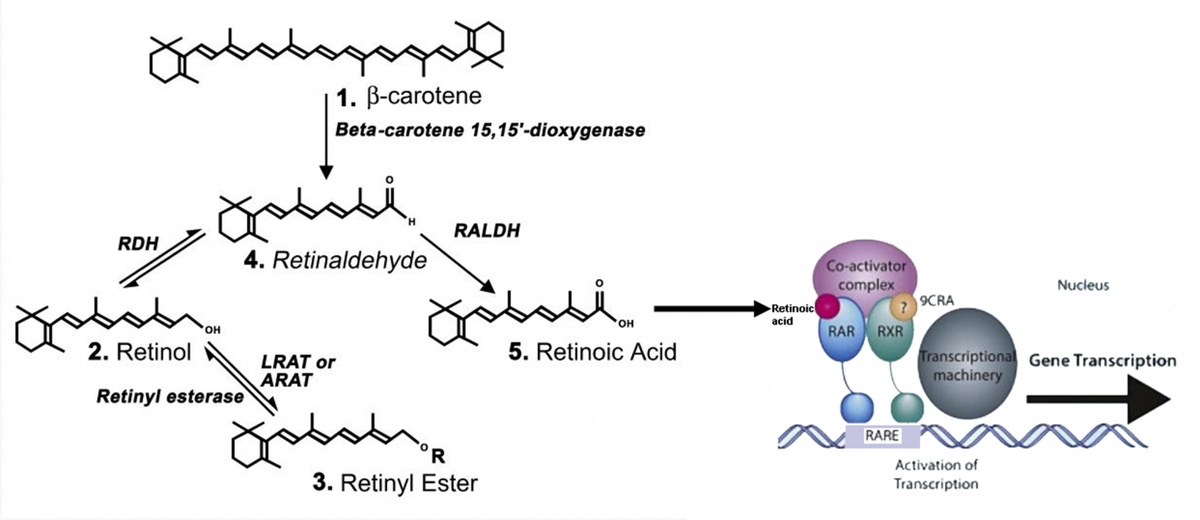
Synthesis from beta-carotene, retinyl ester or retinol of retinoic acid (left) and it activation via retinoic acid receptors of gene expression (right).
We have found that retinoic acid is synthesized in the brain where it has numerous functions, for instance in the hippocampus (controlling birth of new neurons), hypothalamus (mediating seasonal changes in body weight) and pineal gland (regulating day/night changes). We are now using our knowledge of the action of retinoic acid in the brain to protect neurons from dying to develop retinoic acid like compounds that will be therapeutic for motor neurons diseases, with an emphasis on amyotrophic lateral sclerosis (ALS), in which motor neurons die, as well as Alzheimer's disease.
Research Specialisms
- Pathobiology
- Neuroscience
- Molecular Biology
- Cell Biology
- Metabolic Biochemistry
Our research specialisms are based on the Higher Education Classification of Subjects (HECoS) which is HESA open data, published under the Creative Commons Attribution 4.0 International licence.
Current Research
(1) Therapeutic roles of novel RAR ligands (RAR-Ms) for treatment of amyotrophic lateral sclerosis (Azita Kouchmeshky)
Amyotrophic lateral sclerosis (ALS) is a heterogenous progressive motor neuron degenerative disorder for which the exact pathogenesis and effective treatment are lacking. The retinoic acid signalling pathway plays crucial roles in the central nervous system to promote cell survival. We have shown, for instance, that motor neurons express the enzymes that synthesize retinoic acid from vitamin A. It is unexplored however whether it may protect motor neurons in ALS. Thus, the neuroprotective effect on ALS of novel retinoic acid receptor (RAR) ligands, called RAR-Ms, are being investigated. The neuroprotective effect of RAR-Ms is investigated using a series of novel methods that study (i) glutamate-induced excitotoxicity in rat primary cortical neurons, (ii) RNA foci, TDP-43 dislocation, and survival rates motor neuron like cell line NSC-34 stably transfected with C9ORF72 expanded repeat and (iii) assembly and disassembly of stress granules (SGs) in NSC-34 cells transiently transfected with (GFP)-SOD1G93A. Further, we have set up a novel assay to determine how RAR-Ms are distributed after injection into mice. To date we have shown that the lead RAR-M compound, DC645, showed a neuroprotective effect in the excitotoxicity assay, significantly increasing the number of cortical neurons following glutamate treatment and also increased the survival rate of the NSC-34 cells expressing C9orf72 gene. Furthermore, DC645 affects the assembly of SGs in the NSC-34 cells expressing (GFP)-SOD1G93A. Determination of in-vivo distribution of DC645 in the mouse following IP injection showed high levels of accumulation in the spinal cord, as would be needed for ALS treatment. Further investigation of the RAR-Ms continue as a potential ALS therapeutic.
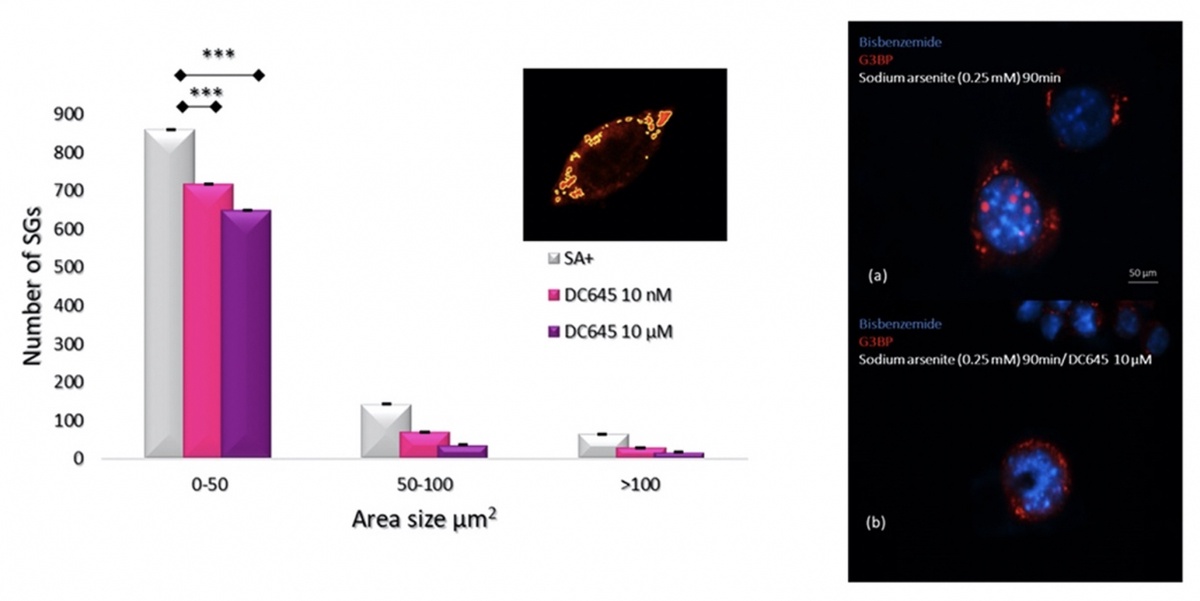
The RAR-M DC645 reduces stress granule numbers in oxidative stress induced NSC-34 motor neuron like cells.
(2) Application of artificial intelligence-driven design of function-directed ligands for selective retinoic acid receptor binding (Jason Clark)
The retinoic acid receptor (RAR) class of nuclear receptor have great future potential for the treatment of neurodegenerative diseases. However the shape and structure of RAR ligands that optimally activate RARs is poorly understood. This project, a collaboration between University of Aberdeen, Durham University and University College London, aims to model RARs to design novel ligands that activate the receptors and understand the triggering routes for RARs for both genomic and non-genomic signalling. A radically different approach is taken to ligand design, to modelling and to understanding binding selectivities to the different RARs. Used is a combination of molecular docking, atomistic molecular dynamics simulations and machine learning techniques, to move beyond static 2D or 3D ligand descriptors and develop complex Quantitative Structure–Activity Relationship (QSAR) models which incorporate dynamics alongside shape and chemical selectivity. Further, the techniques employed include an AI approach to ligand design including the use of domain-specific technologies such as DeepChem and more generic tools such as Keras and TensorFlow. From the chemical and biological side, synthetic retinoids predicted from the above work are prepared and applied in a variety of assays for RAR activity such as transcriptional activity, as well as non-genomic signalling via a variety of kinases and control of protein translation using cell lines and primary neural cells.
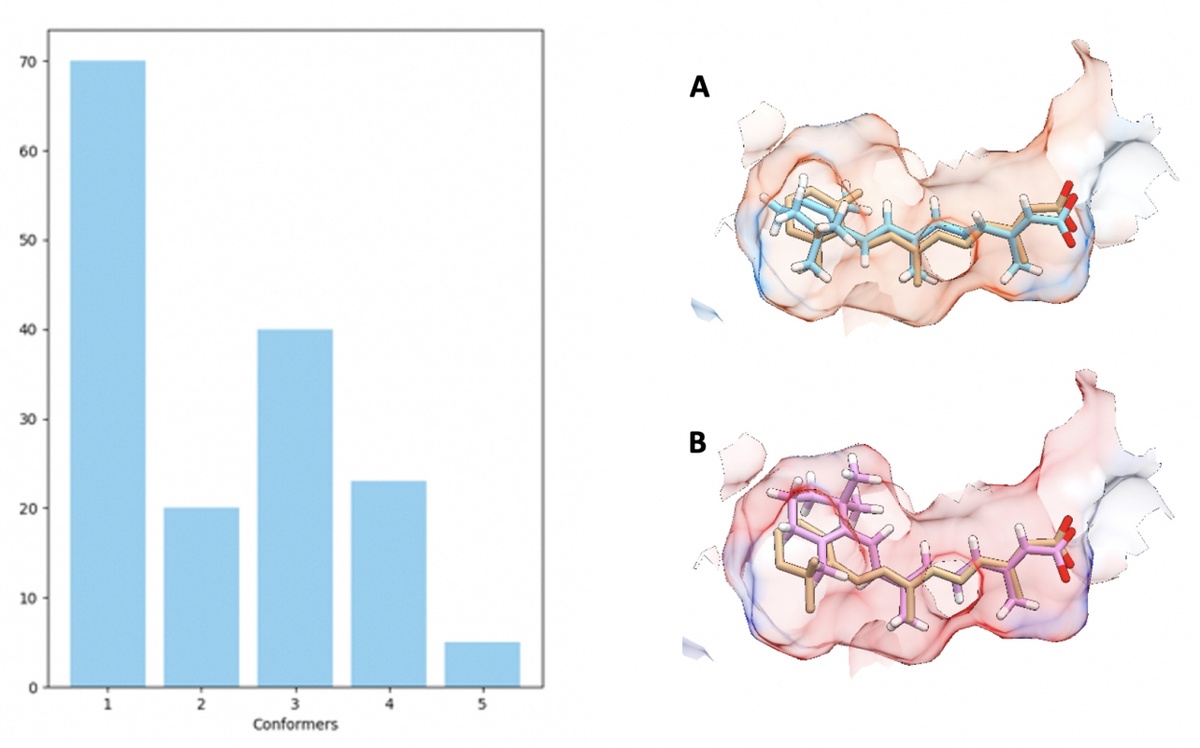
An Algorithm-determined conformational clustering histogram (left) summarising the distribution of the conformations of retinoic acid that were observed docking with RARgamma at a 1.2 Å RMSD threshold (left). Overlaid comparison of original bound ligand state (orange) with key conformers selected by highest frequency (A) and top ranking by ChemScore (B).
(3) Development of multi-dimensional approaches to understand how retinoic acid receptors control neuronal behaviour (Peter McCaffery)
We have a unique approach to study retinoic acid receptor (RAR) ligands, highlighting their multi-dimensional action as regulators of transcription (genomic action) and modulators of rapid pathways in the cytoplasm (non-genomic actions). We studied new RAR ligands called RAR-modulators developed by Nevrargenics Ltd. These ligands have a diverse range of actions on genomic/non-genomic pathways and varied capacity to induce major morphological change in neurons. This company developed these RAR ligands for treatment of neurodegenerative disease (NGD), with a current focus on amyotrophic lateral sclerosis. The multi-modal analysis we have taken has already identified a lead compound and the proposed project will expand the approach to help identify optimised RAR-modulators for varied NGDs.
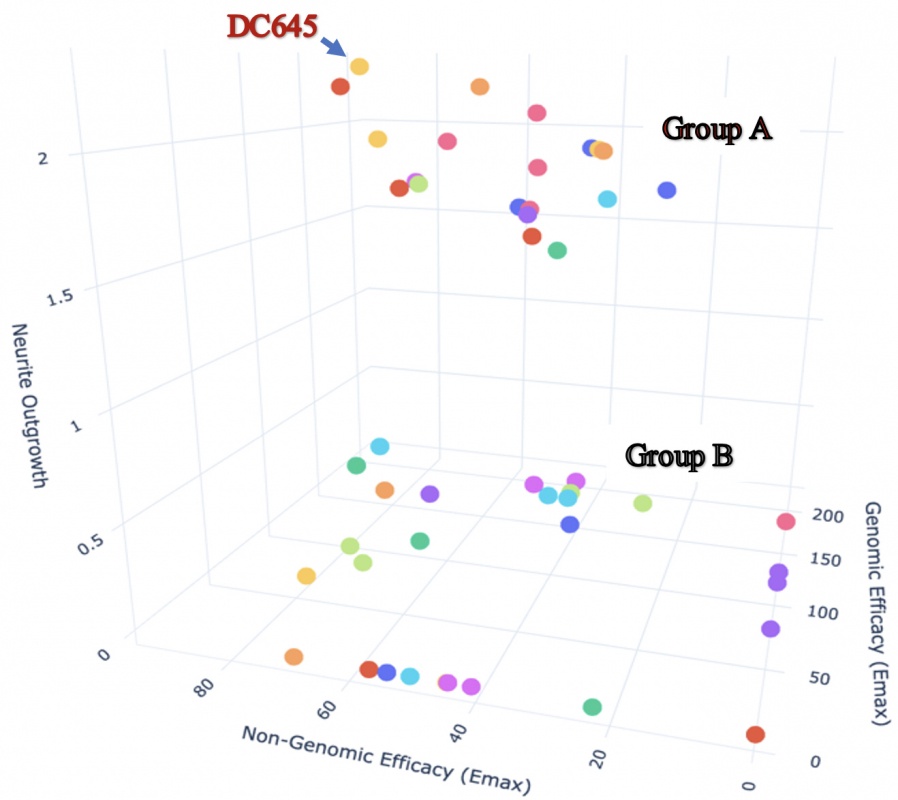
3D analysis of 10 nM RAR-M action on SH-SY5Y cells plotting genomic (transcriptional), non-genomic (ERK activity) and neurite outgrowth on the 3 axes. High genomic/non-genomic activity is necessary for high neurite activity (Group A) but a second group with these activities (Group B) almost totally lack neurite outgrowth-inducing activity.
(4) How vitamin A homeostasis may be controlled by the hypothalamus (Peter Imoesi)
Vitamin A (retinol) occurs in animals as esters of higher fatty acid which is an essential constitute of mammalian diet. Basically vitamin A is derived from carotenoids which are plant pigments or the liver of animals. Essentially dietary retinol is transported in circulation and bound to retinol binding protein (RBP). Subsequently, with the aid of RBP receptor stra6, cells are able to recruit and transport retinol to the cytosol. In the cytosol retinol is bound to cellular retinol binding protein (CRBP) and through various enzymatic processes retinol is converted to retinoic acid in the nucleus to initiate gene transcription. Retinol is maintained at a precise concentration of 1µM in plasma despite the slight increase of dietary intake. However, how the body is able to maintain this concentration is relatively unknown. Previous research as implicate the hypothalamus as the central control of body homeostasis for instance glucose. My research is to determine how vitamin A (retinol) homeostasis is controlled by the hypothalamus. To investigate this, rats will be injected with retinoic acid (RA) the active metabolite of retinol to the hypothalamus using a stereotactic procedure and subsequently systemic increase or decrease of RA will be measured over time.
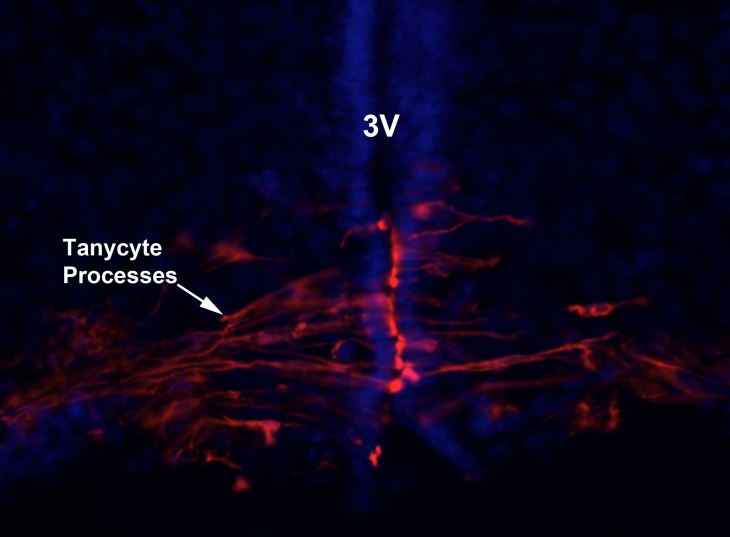
Tanycytes lining the wall of the third ventricle (3V) of the hypothalamus may be a key cell in the control of vitamin A homeostasis
Collaborations
Bill Blaner (Columbia University)
Siddharthan Chandran (University of Aberdeen)
Dave Chisholm (Nevrargenics)
Iain Greig (University of Aberdeen)
Bhuvaneish Selvaraj (University of Edinburgh)
Andy Whiting (Durham Univerity)
Supervision
We have been very fortunate to have had a series of excellent students, undergraduate and postgraduate, as well as post-doctoral fellows, who have greatly contributed to our research. Many stay in contact and we have been tremendous to see their careers advance in research, teaching, communication, industry and varied others, as you will see below.
Summer Students
Miss Jessica Fattal
Last degree
Bachelors of Science (Neuroscience and Psychology)

Current position
Research Assistant at Michigan State Univ.
Email address
Research interest
Investigating the physiological mechanisms underlying various forms of mental illness
Miss Francesca Moramarco
Last degree
MSci student
in Neuroscience with Psychology with Industrial Placement

Current position
MSci Student, University of Aberdeen
Email address
Research interest
Translational neuroscience and Neurobiology
Ms. Danny Schnitzler
Last degree
MSci Biomedical Science (Physiology)

Current position
PhD student, University of Edinburgh
Email address
Research interest
Neuroendocrinology
sex hormones; prenatal programming
Dr. Karen Jane Thompson
Last degree
PhD in Neuroscience, University of Aberdeen

Current position
Associate Medical Writer, CMC Connect
Research interest
Wide ranging, from retinoic acid in the pituitary gland, glutamate receptor and other channels, in mechanosensory nerve terminals, muscarinic acetylcholine receptors in Alzheimer's disease, and the potential of the endocannabinoid system in neurodegenerative diseases.
Mr Timothy Nehila
Last degree
B.A. in Biology from Case Western Reserve University

Current position
Post-Baccalaureate Researcher in the lab of Dr. Agbor-Enoh at the National Institutes of Health
Email address
Research interest
transplantation genomics
Dr. Marta U. Wołoszynowska-Fraser
Last degree
PhD in translational neuroscience - University of Aberdeen

Current position
Postdoctoral Visiting Fellow at the National Institute on Aging (Baltimore, USA)
Email address
Research interest
My current research interest includes retinoic acid signalling system differences in the hippocampus of aged animals with and without memory impairment
Masters Students
Mr Shakil Khan
Last degree
MRes Drug Discovery
Current position
PhD student at Aston University
Email address
Research interest
Role of ion channels in disease states
Mr. Sudheer Nunna
Last degree
Master of Research (MRes)

Current position
Analytical Scientist, Lonza Biologics Pte. Ltd (Singapore)
Email address
Research interest
Proteomics
Ms. Sylwia Matz
Last degree
MSc Drug Discovery and Development

Current position
Global Clinical Trial Manager
Email address
Research interest
Project Management, Clinical Research, Oncology
Grace Okpara Chidimma
Last degree
MSc Clinical Pharmacology
Doctor of Veterinary Medicine (DVM)

Current position
CEO Ultra Salem Sphare
Veterinary Services and Consultancy. Kaduna Nigeria
Email address
Research interest
Pharmacogology
Animal health and Production.
Mr Obuchinezia Anyanwu
Last degree
MSc Drug Discovery and Development

Current position
Junior Medical Writer at Aparito
Email address
Research interest
neuromuscular diseases
Veronika Lavickova
Last degree
MSc Molecular Medicine

Current position
Lab Project Set-up Coordinator
Email address
Research interest
Clinical Research
PhD Students
Dr. Reem Bakr Busaeed
Last degree
PhD

Current position
Assistant Professor in Anatomy, College of Medicine, Jeddah
Email address
Research interest
Bidirectional interaction between endocannabinoid and retinoid signalling pathways in the brain, plus Anatomy and clinical work
Dr Sonia Elena Nanescu
Last degree
PhD

Current position
Scientific review specialist (contractor) at NCCIH( National Center for Complementary and Integrative Health) /NIH (National Institute for Health)
Email address
Sonia.nanescu.sn@gmail.com
Research interest
Neuronal repair and regeneration, remyelination, vitamin A
Dr Peter Ikhianosimhe Imoesi
Last degree
PhD

Current position
Research Fellow, University of Aberdeen
Email address
Research interest
The role of neural connection and debilitating diseases, learning and memory, neurodegenerative diseases, homeostasis of biomolecules e.g. retinoids and obesity.
Dr Jemma Ransom
Last degree
PGCE (Chemistry) University of Leeds

Current position
Company Director, Little Learners Durham & Darlington
Email address
Research interest
I left science at the end of my PhD and I now run an educational company in Darlington specialising in promoting literacy amongst the under 5's. I'm a mum of a 1 year old and a 4 year old so I guess my primary research interest at present is keeping the peace!
Dr Anna Ashton
Last degree
PhD

Current position
Post-doctoral Researcher, University of Oxford
Email address
Research interest
Molecular mechanisms underlying the circadian clock
Dr Thabat Khatib
Last degree
PhD in Translational Neuroscience

Current position
Postdoctoral Research Fellow at the Hebrew University of Jerusalem
Email address
Research interest
Neurodegenerative Diseases, Neuroplasticity, Retinoic Acid, Evolutionary Genetics & Epigenetics, Stem Cells, Drug Discovery & Translational Neuroscience.
Mrs Victoria Gorberg
Last degree
BSc in Molecular Biology

Current position
PhD student
Email address
Research interest
Tourette syndrome, Cannabinoids.
Ms Azita Kouchmeshky
Last degree
MSc (Neuropharmacology)
Pharm D. (Doctor of Pharmacy)

Current position
PhD student Translational Neurosciences
Email address
Research interest
Disturbance in normal neuroprotective regulatory signalling pathways in the CNS associated with pathophysiology of neurodegenerative diseases
Dr Timothy Goodman
Last degree
PhD in Neuroscience (University of Aberdeen)

Current position
Senior Laboratory Research Scientist, The Francis Crick Institute
Email address
Research interest
Neuronal circuits underlying multisensory integration in the superior colliculus
Research Fellows
Dr. Kirst Shearer
Last degree
PhD

Current position
NRS Cancer Research Network - North & East Manager
Email address
Research interest
Retinoic acid and adult hippocampal and hypothalamic function
Dr. Patrick Stoney
Last degree
PhD
Medical Sciences, University of Aberdeen

Current position
Staff Scientist, Yamamoto Unit, Okinawa Institute of Science and Technology (OIST), Okinawa, Japan
Email address
p.n.stoney@gmail.com
Research interest
I am currently working on the regulation of mRNA stability by the CCR4-NOT deadenylase complex
Funding and Grants
Wellcome, BBSRC, Tenovus, Autism Speaks, The Royal Society and The Royal Society of Edinburgh
- Teaching
-
Teaching Responsibilities
I have a Postgraduate Certificate in Higher Education Learning & Teaching and am a Fellow of the Higher Education Academy. Presently I am an external examiner for the School of Biology, University of St Andrews
I teach in various courses in Aberdeen including:
BScMedSci SSCII and SSCI
SM2501 Research Skills for Medical Sciences
SM2001 Foundation Skills for Medical Sciences
BI25M7 Energy for life labs
BT5007 Industrial Placement.
BM4004 Advanced Molecules. Membranes and Cells lectures and practicals
BM4010 Stem Cells and Regeneration
BC4014 Biochemistry Option 1
MT5024 Mol Pharmacol - Publications
-
Page 1 of 10 Results 1 to 10 of 93
Synthetic retinoids for the modulation of genomic and non-genomic processes in neurodegenerative diseases
ACS Omega, vol. 10, no. 22, pp. 23709–23738Contributions to Journals: ArticlesNeuroprotective effects of ellorarxine in neuronal models of degeneration
Frontiers in Neuroscience, vol. 18, 1422294Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.3389/fnins.2024.1422294
- [OPEN ACCESS] http://aura.abdn.ac.uk/bitstreams/6fe39f24-ef9e-4c0f-9aad-2995b9db797b/download
- [ONLINE] View publication in Scopus
A Vitamin Treatment for Motor Neurone Disease
Lipophilic vitamins in health and disease. Tappia, P. S., Shah, A. K., Dhalla, N. S. (eds.). Springer Nature, pp. 275-290, 16 pagesChapters in Books, Reports and Conference Proceedings: ChaptersRetinoic acid regulation of homoeostatic synaptic plasticity and its relationship to cognitive disorders
Journal of Molecular Endocrinology, vol. 72, no. 1, e220177Contributions to Journals: Review articles- [ONLINE] DOI: https://doi.org/10.1530/JME-22-0177
- [ONLINE] View publication in Scopus
Control by the brain of vitamin A homeostasis
iScience, vol. 26, no. 8, 107373Contributions to Journals: ArticlesLoss of Neuron Navigator 2 Impairs Brain and Cerebellar Development
Cerebellum, vol. 22, no. 2, pp. 206-222Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1007/s12311-022-01379-3
- [OPEN ACCESS] http://aura.abdn.ac.uk/bitstreams/59ddacd5-d7a8-448b-ad69-d1cb59981ec5/download
- [ONLINE] View publication in Scopus
Retinoic Acid Signalling in the Pineal Gland Is Conserved across Mammalian Species and Its Transcriptional Activity Is Inhibited by Melatonin
Cells, vol. 12, no. 2, 286Contributions to Journals: ArticlesMotor-like Tics are Mediated by CB2 Cannabinoid Receptor-dependent and Independent Mechanisms Associated with Age and Sex
Molecular Neurobiology, vol. 59, pp. 5070-5083Contributions to Journals: ArticlesA Bioluminescence Reporter Assay for Retinoic Acid Control of Translation of the GluR1 Subunit of the AMPA Glutamate Receptor
Bioluminescence: Methods and Protocols, Volume 1. Kim, S. (ed.). 4 edition. Humana Press, pp. 197-207, 11 pagesChapters in Books, Reports and Conference Proceedings: Chapters (Peer-Reviewed)- [ONLINE] DOI: https://doi.org/10.1007/978-1-0716-2453-1_15
Differential retinoic acid signaling in the hippocampus of aged rats with and without memory impairment
eNeuro, vol. 8, no. 5, ENEURO.0120-21.2021Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1523/ENEURO.0120-21.2021
- [OPEN ACCESS] http://aura.abdn.ac.uk/bitstreams/9386a196-213c-4186-b2cc-c4fca7e40506/download
- [ONLINE] View publication in Scopus
