Some of our ongoing antibody generation projects are showcased here
- New and efficacious bi-specific drugs for NASH
-
Knowledge Transfer Partnership with Elasmogen Ltd
Non-alcoholic steatohepatitis (NASH) is a progressive form of Non-Alcoholic Fatty Liver Disease (NAFLD) characterised by chronic inflammation and liver damage due to fat accumulation. NASH is associated with an unhealthy diet and lack of physical activity and, although it is a silent chronic disease with few or no symptoms (tiredness, and discomfort in the upper right side of the abdomen), it can develop overtime into more serious conditions, such as liver cirrhosis or even cancer. Critically, to date, there is no specific medication approved by Food and Drug Administration (FDA) for the treatment of this devastating disease, which is now the most common chronic liver condition in Western populations, and with patient numbers growing rapidly, the market is expected to rise towards £20 B by 2026.
In chronic liver diseases including NASH, hepatic stellate cells (HSCs) get activated into myofibroblasts and generate scarring through the accumulation of extracellular matrix proteins. It has been shown that stimulating apoptosis of activated HSCs can resolve fibrosis and enhance the liver's response to chronic damage. In previous studies, we have generated a panel of antibody fragments that bind specifically to Synaptophysin, a plasma membrane protein expressed in activated HSCs. Synaptophysin stabilises the vesicles which are involved in the transport of cellular contents (extracellular matrix proteins). Interestingly, binding domains that recognise an intra-vesicular loop of Synaptophysin, which is exposed on the surface of HSCs during biochemical transport, enables antibody binding and internalisation during vesicle recycling.
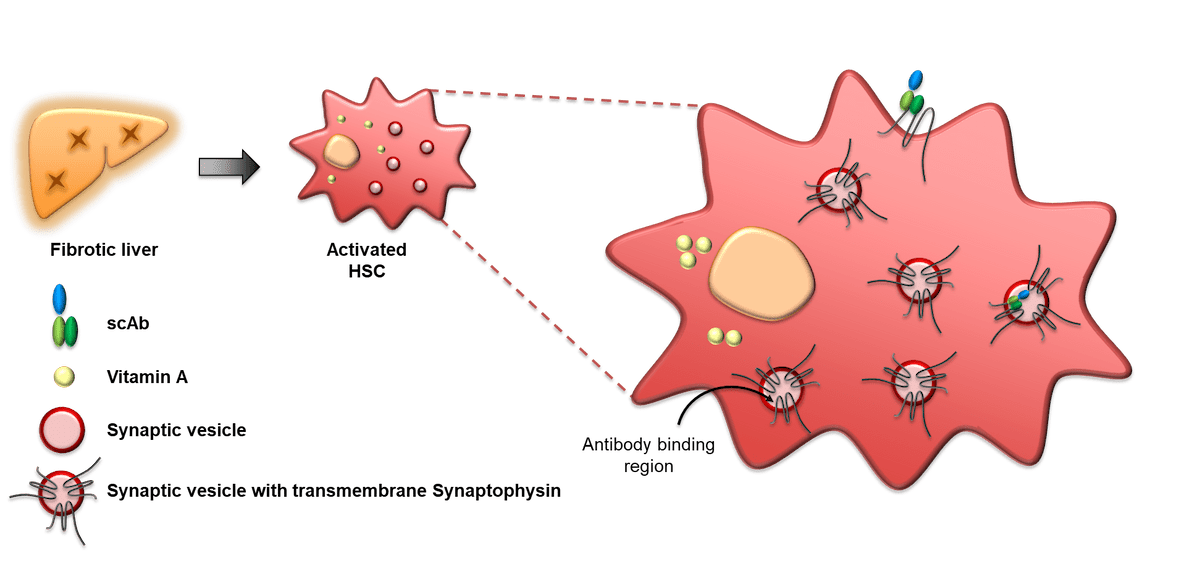
To further increase the specificity of HSC targeting the KTP project will develop bispecific drugs using a combination of the existing synaptophysin binders and HSC specific soloMERs isolated from Elasmogen Ltd's single-domain, drug-libraries.
By combining the specificity of Elasmogen's soloMERs with the internalisation activity of synaptophysin antibodies, we are creating a novel class of potent bispecific drugs that are tailored specifically for liver disease.
- Novel anti-infective biologics
-
The world is facing an anti-microbial resistance (AMR) armageddon, forecast to cost $100 tn in global GDP and 10 million deaths by 2050. Accepted now as a 'complex global challenge', tackling AMR requires international scientists, pharma companies and funding organisations to work together to develop a combination of innovative drugs and efficient diagnostics. In the SBF we are generating novel biologics-based lead molecules for the rapid diagnosis (point-of-care settings) and treatment of drug resistant, difficult to treat G-ve bacterial infections and life-threatening fungal infections. We are also involved in a multi-platform Covid-19 project that has produced almost 70 human antibody binders to 4 key viral proteins. These binders have been made available to a number of academic and commercial partners and are available to others on request.
Away from Covid-19 our anti-infective projects focus on novel drug discovery approaches such as antibody-mediated targeting of essential proteins required for microbial viability in vivo or the blocking of key virulence pathways that contribute towards host pathogenicity during an infection. Such strategies aid the clinical transition towards narrow-spectrum pathogen specific drugs (vs broad spectrum antimicrobials), with the possible consequence of slowing down the evolution of drug-resistant microorganisms. The adoption of narrow spectrum antimicrobials has been hampered by the lack of rapid and sensitive diagnostics tests that can accurately detect (ideally early in the infection) the disease-causing pathogen. To address this issue, some of our pathogen specific, high affinity/sensitivity antibodies that recognise disease specific biomarkers have been tested in biological samples and their potential to be used in accurate, point-of-care, narrow spectrum diagnostic test systems demonstrated.
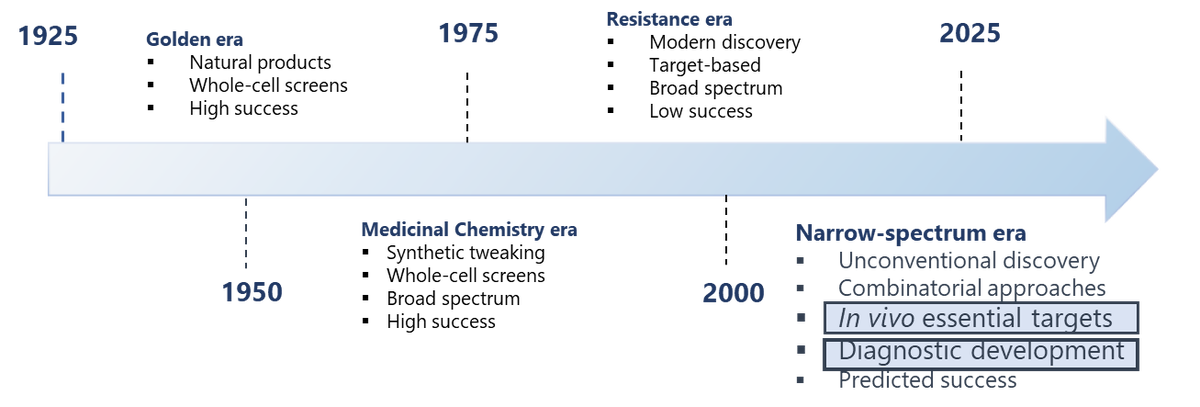
*Figure adapted from Brown & Wright, Nature 2016
High sensitivity sheep antibodies to block cell-cell communication in Pseudomonas aeruginosa
- Quorum sensing systems co-ordinate pathogenesis and control the expression of virulence factors and biofilm formation
- We have generated nanomolar sensitivity mAbs towards 'antigenically inert' Homoserine Lactones (HSL) compounds through sheep immunisation
- Lead mAbs have demonstrated a protective role in nematode slow killing assay
- mAbs significantly increased mice survival in a P. aeruginosa lung infection model
- The ability to detect low nM levels of HSL molecules in biological matrices illustrated their potential as a possible diagnostic immunoassay
Alkylquinolone (AQ) antibody-based detection of Pseudomonas aeruginosa in respiratory diseases
- AQs are a second class of quorum sensing signalling molecules as well as reliable biomarkers for P. aeruginosa detection in Cystic Fibrosis lungs
- As part of a collaboration with the University of Nottingham, we have generated nanomolar sensitivity AQ antibodies to detect the pathogen in early pulmonary infections enabling prompt treatment
BBSRC EASTBIO Doctoral Training Partnership PhD programme 2020-2024
- Aims to understand the pathogenesis of life threatening Burkholderia cenocepacia infections using blocking antibodies
- Develop novel chemistry scaffolds to untangle complex aspects of AMR
- Immunodiagnostics and therapeutics for neurodegenerative diseases
-
Commercial partner - TauRx Pharmaceuticals Ltd*
Dementia affects 50 million people worldwide with Alzheimer's disease accounting for 50-60% of all cases. This number is expected to triple by 2050 and despite continuous scientific effort and the commitment of several billion USD in clinical development, there are no disease modifying drugs (only symptom modifying) for AD approved by the FDA or EMA. We are beginning to form the opinion that this may be because treatments start after the damage has already occurred. Studies show that pathophysiological changes in the brain begin several decades before AD symptoms. However, AD diagnosis is still fully reliant on the presentation of clinical symptoms and further highlights the need to develop validated biomarker based diagnostic test systems that can detect early signs of neurodegeneration, disease onset and monitor its progression and/or response to therapy.
According to the tau hypothesis, abnormal aggregation of tau protein leads to the formation of tangles in the neurons. Once initiated, this tau aggregation process continues, spreading the aggregation cascade into previously healthy nerve cells. In Alzheimer's disease tau tangles first appear in nerve cells critical for memory and these are the first cells to be lost. The spread of cell death through the brain is closely linked to the systematic spread of these tau tangles.
© TauRx Pharmaceuticals Ltd, with permission.
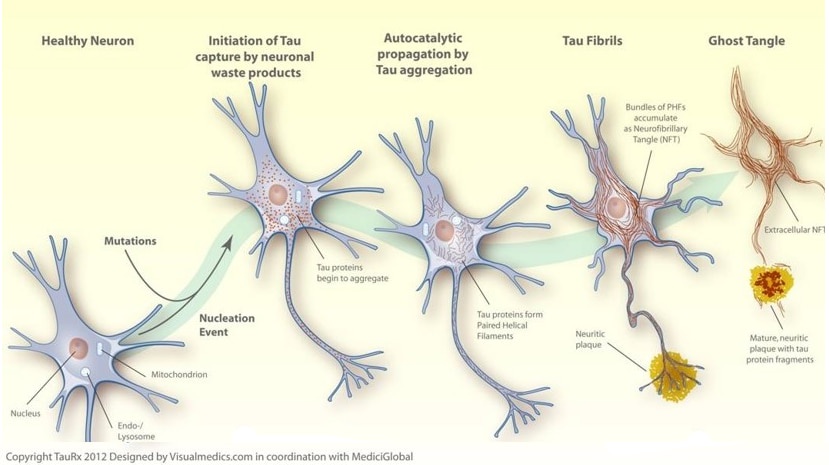
The SBF, in partnership with TauRx Pharmaceuticals, is developing a diverse panel of high affinity monoclonal antibodies targeting specific regions of the tau protein. We are turning these mAbs into powerful biomarker-based diagnostics and disease modifying therapies with applications for many related neurodegenerative diseases termed tauopathies.
*TauRx Pharmaceuticals Ltd is parent of TauRx Therapeutics Ltd and Wista Laboratories Ltd.
