Research
Oxide Ion Conductors
Solid oxide fuel cells (SOFCs) are electrochemical devices able to generate electricity from renewable energy sources, with high efficiency and low emissions of pollutants. However, oxide ion transport in most commercially available solid electrolytes is enabled only at high working temperatures (> 700 °C), thus making the commercialization of SOFCs particularly challenging. The high operating temperature reduces the components' durability, results in slow start up times and the necessitation of expensive materials for seals and interconnects. In order to overcome these issues, it is desirable to develop novel electrolyte materials with significant oxide ion conductivity at intermediate temperatures (300 - 600 °C). Ionic conduction in solid oxides is strongly related to the crystal structure, and as a result the discovery and characterization of novel oxide ion conductors in various structural families has attained significant attention from researchers. We have been investigating hexagonal perovskites Ba3M'M''O8.5 (M'M'' = VW, NbMo, NbW) which exhibit highly disordered crystal structures and state-of-the art conductivity.

Mixed Proton/oxide ion conductors
We are also interested in mixed ion conductors, specifically mixed oxide-ion and proton-conductors. Such dual ion conductors have been proposed as a new class of electrolyte for intermediate temperature ceramic fuel cells, as they exhibit low ohmic resistance without external gas humidification. We have been investigating the electrical and structural properties of Ba7Nb4MoO20, a cation-deficient 7H hexagonal perovskite derivative formed by an intergrowth of palmierite layers and 12R perovskite blocks. Ba7Nb4MoO20 supports remarkable oxide ion and proton conductivity with excellent chemical and electrical stability over a range of pO2. The ionic conduction properties of Ba7Nb4MoO20 are linked to its distinct disordered crystal structure. Ba7Nb4MoO20 supports pure ionic conduction with high proton and oxide ion conductivity at 510 °C (the bulk conductivity is 4.0 mS cm-1) and hence is an exceptional candidate for application as a dual-ion solid electrolyte in a ceramic fuel cell which will combine the advantages of both oxide ion and proton conducting electrolytes. We are currently performing chemical doping studies to further enhance the conductivity.
Magnetic Materials
The recent report of high temperature superconductivity in oxypnictides such as LnFeAsO1-xFx with superconducting transition temperatures (Tcs) up to 55 K has led to a rapid expansion in the research of oxypnictide materials. We have recently discovered the counterpart, colossal magnetoresistance (CMR) in Mn2+ based oxypnictides NdMnAsO1-xFx, so that both manifestations of correlated electronic behaviour are now found in these new materials. Colossal magnetoresistant materials exhibit a large change (> 90%) in their electronic resistivity upon application of a magnetic field and have application as magnetoresistive sensors and spintronic devices in which electron spins are used to process information. We are currently investigating more complex cation ordered oxypnictides such as Sr2Mn2CrAs2O2 which exhibit spin reorientation magnetic transitions upon cooling.

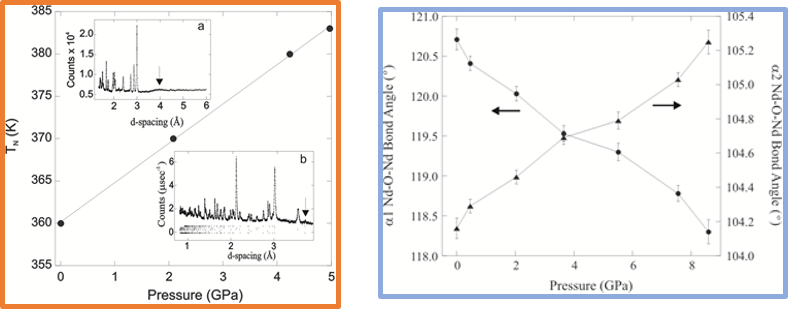
High Pressure
Variable pressure experiments can induce dramatic changes in the crystal structure and physical properties of materials. The nuclear and magnetic lattice can be studied using high pressure neutron diffraction, where pressure is applied using a Paris-Edinburgh press and an appropriate anvil to achieve pressures up to 23 GPa. At the University of Aberdeen we have designed experiments where pressure is used to manipulate the magnetic transitions of magnetoresistive materials and tune the disorder within hexagonal ionic conductors. For example, the antiferromagnetic transition of the colossal magnetoresistant material NdMnAsO0.95F0.05 is enhanced from 360 K to 383 K with a pressure of 4.97 GPa. And high pressure studies show that, while no structural transitions are induced, the material violates Bloch's rule which suggests that the phase is on the verge of a localised transition. The oxide ion conductor Ba3MoNbO8.5 also undergoes an interesting transformation, as between 0 - 6 GPa at 300 K, a structural rearrangement within the oxide ion lattice occurs which causes a redistribution of vacancies and the disorder within these layers. At ambient pressure, the crystal structure is a hexagonal hybrid between the 9R perovskite (A3B3O9) and palmierite (A3B2O8) unit cells. Applied pressure enhances the 9R character, as the palmierite contribution steadily decreases until ~ 5.2 GPa, after which the palmierite is eliminated and the structure becomes fully 9R.
Biomaterials
We are interested in understanding how the properties of various calcium phosphate-based biomaterials can be modified by ionic substitutions or by modified synthesis methods. The solubility, osteoconductivity and osteoinductivity of caclium phosphates can all be affected and optimised by modifying the physicochemical properties. We have particular interests in substitution of phosphate in calcium phophate phases for silicate or carbonate and how this can control properties. This opens up opportunities to study co-substitution of mono- or tri-valent cations for calcium, and has led to a number of studies in our group to explore new compositions. Some of these can be used to produce scaffolds and others calcium phosphate cements, both of which have potential application as bone replacement materials. We have the ability to study the biological response to such materials at the Institute of Medical Sciences at our Foresterhill campus.

Carbon Capture and Utilisation
This is an emerging theme in our group and results from the study of carbonated materials which can be adapted to optimise compositions that then can incorporate carbonate. We can study these materials using the standard solid state equipment, but also using thermogravimetry couples with mass spectrometry, so we can optimise temperatures of carbonate uptake, for example. Materials can be modified by using targetted ionic subsitutions to create solid solutions for study.
This work complements projects in Engineering and in other parts of Chemistry and is in collaboration with the School of Biological Sciences.

Single-crystal diffraction
The ultimate characteriation technique for solids locates atoms in space and defines the symmetry relations between them to unprecedented precision. Two interesting examples of recent work are hybrid perovsites consisting of a network of corner-sharing ACl6 (A = K, Rb, Cs) octahedra encapsulating C5H14N22+ cations and the conformations of 'bent' liquid-crystal precursors, where subtle differences in molecular conformations play a critical role in determining properties.

