
PhD CPsychol AFBPsS
Personal Chair
- About
-
- Email Address
- s.maclennan@abdn.ac.uk
- Telephone Number
- +44 (0)1224 438125
- Office Address
Academic Urology Unit Health Sciences Building (2nd Floor) University of Aberdeen Foresterhill Aberdeen AB25 2ZD
- School/Department
- School of Medicine, Medical Sciences and Nutrition
Biography
Co-director Aberdeen Cancer Centre
I am a psychosocial oncologist and a health psychologist with expertise in qualitative research methodology. I pursue an interdisciplinary approach building multi-stakeholder working in cancer care and have established global partnerships of academics, patients, non-profit organisations, professional organisations and policy makers.
I am Deputy Director of the Academic Urology Unit, Director of Operations for UCAN (Urological CANcer charity) and hold an honorary contract with NHS Grampian. I am an Honorary Research Fellow, Birkbeck University of London. I am a Chartered Psychologist and Associate Fellow of the British Psychological Society, and a registered health psychologist (Health & Care Professions Council).
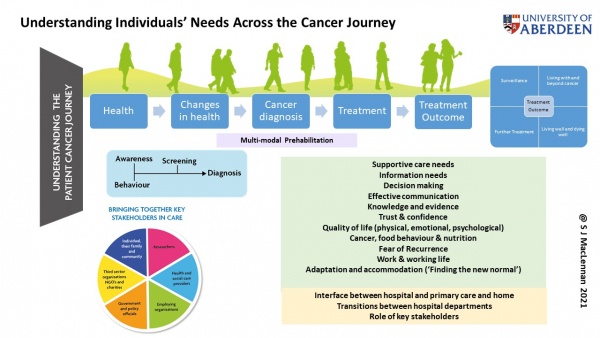
Current projects include:
Stakeholder engagement in the design and implementation of cancer care:
ICANBE – Managing Fear of Cancer Recurrence
EVOLVE – Giving cancer patients a meaningful voice within the design and delivery of clinical practice guidelines
ICANTREATNEPAL / ICANTREATINDIA – Increasing participation in cancer screening and treatment in India & Nepal (International Global Health Network)PARTNER –Men’s prostate cancer treatment preferences
OPTIMA – Optimal treatments for patients with solid tumours in Europe through AIPIONEER – Enhancing prostate cancer diagnosis and treatment through the power of big data in Europe
Example of Impact:

I am to keen to supervise PhD students interested in working in cancer survivorship and supportive care.
Memberships and Affiliations
- Internal Memberships
-
University Committees:
Lead - Collaborations and Projects - IAHS Executive Committee
Previous Committees:
Chair - Institute of Applied Health Sciences (IAHS) Research Strategy Group
Chair - Institute of Applied Health Sciences (IAHS) Health, Safety & Well-being Committee - External Memberships
-
Current External Membership:
Trustee: Lobular Breast Cancer UK (2023 onwards - )
Member - European Cancer Organisation Steering Committee for Survivorship and Quality of Life (2021 onwards)
Member - European Cancer Organisation Survivorship Focused Topic Network (2021 onwards)
Member - European Cancer Organisation Workforce Focused Topic Network (2021 onwards)Member – Patient Advocacy Group, European Association of Urologists (2018 - onwards)
Previous External Membership:
Member – Executive Committee, British Psychosocial Oncology Society (2016 - 2022)
Member – National Cancer Research Institute –Living With and Beyond Cancer Late Effects Workstream (2019 -2021)
Member – Breast Cancer Now, Grants Award Committee (2018 – 2021)
Member – ESRC Peer Review College (2017 – 2020)Member – MRC Strategic Skills Panel (2013 - 2017)
Member – Grants Award Panel, Carnegie Trust
Member – Executive Committee, Division of Health Psychology Scotland (2016 - 2017)
External Examiner (MSc) MSc Health Psychology, University of Westminster (2008 - 2011)
Member - BPS working party on ‘Work and Health’
Elected Member – Training Committee, Division of Health Psychology (2006 - 2008)
Elected Member - Executive Committee, Division of Health Psychology (2006 – 2008)
Assessor - Board of Assessors, Division of Health Psychology (2002 - 2008)
Examiner – Paper 4 (Health Psychology), Qualifying Examination (2002 – 2012)
Member - BPS working party on ‘Psychological Debriefing’(2001 - 2002)
Latest Publications
Shared Decision-Making in Urology: The SHINE-URO Consensus Framework
European Urology FocusContributions to Journals: ArticlesEffect of Dietary Interventions on Body Composition and Quality of Life in Stomach Cancer Survivors after Gastrectomy: A Systematic Review
Journal of Gastrointestinal Cancer, vol. 57, 11Contributions to Journals: Review articlesPrevalence and correlates of diagnosed and undiagnosed diabetes mellitus among adults in rural Uganda during the COVID-19 pandemic: an evaluation of a community-based screening program
International Health, vol. 18, no. 1, pp. 17-25Contributions to Journals: ArticlesImpact of the COVID-19 pandemic on quality of life of adults with diabetes in rural Uganda: a cross-sectional survey
International Health, ihaf112Contributions to Journals: ArticlesCorrigendum to “AGREE-II appraisal of lung cancer management clinical practice guidelines by the OPTIMA consortium”. [Lung Cancer 205 (2025) 108610] (Lung Cancer (2025) 205, (S0169500225005021), (10.1016/j.lungcan.2025.108610))
Lung Cancer, vol. 206, 108659Contributions to Journals: Comments and Debates- [ONLINE] DOI: https://doi.org/10.1016/j.lungcan.2025.108659
- [ONLINE] View publication in Scopus
- Research
-
Research Overview
I am a psychosocial oncologist and a health psychologist with expertise in qualitative research methodology. I pursue an interdisciplinary approach building multi-stakeholder working in cancer care and have established global partnerships of academics, patients, non-profit organisations, professional organisations and policy makers. Key to this is an understanding of processes within the delivery of routine clinical care. My research expertise includes process evaluation.
My research strategy focuses on understanding the impact of cancer and treatment on individual’s lives and methods for involving different stakeholder groups, particularly patients, in the design and delivery of care.
Research Areas
Applied Health Sciences
Nutrition and Health
Psychology
Research Specialisms
- Health and Social Care
- Applied Psychology
- Health Psychology
- Oncology
Our research specialisms are based on the Higher Education Classification of Subjects (HECoS) which is HESA open data, published under the Creative Commons Attribution 4.0 International licence.
Current Research
I currently have eleven funded projects (total awards £5,644,624) across three themes:
Stakeholder engagement in the design and implementation of cancer care (Developed and Lead Projects):
- ICANBE – Fear of Cancer Recurrence
- EVOLVE – Giving cancer patient’s a meaningful voice within the design and delivery of clinical practice guidelines across Europe
- PIONEER – Enhancing prostate cancer diagnosis and treatment through the power of big data in Europe; €12,000,000 EU grant (Lead for Aberdeen)
- ICANTREAT – Increasing participation in breast cancer screening and treatment in Nepal and in Uganda; screening and treatment inequalities in India (International Global Health Research Group)
The design and implementation of better cancer care (Co-applicant):
- OPTIMA – Optimal treatments for patients with solid tumours in Europe through AI
- PARTNER – Men’s prostate cancer treatment preferences
- ICAN – Improving understanding of Urological cancer (Cancer Research Group)
- RCOS – Kidney Cancer core outcome set
The design and delivery of care and after care - Urology and other long-term conditions (Co-applicant)
- SIMS LTFU – Surgical management of female stress urinary incontinence
- CATHETER II – Understanding the patient experience of care; embedded qualitative study within a clinical trial to look at Catheter washout policies
- PACFIND – Patient-centred care for Fibromyalgia
Funding and Grants
£1,185,670 Arthritis UK (2019 – 2024). Patient-centred Care for Fibromyalgia: New pathway Design (PACFIND). MacFarlane, G [PI], Maclennan SJ et al. [co-applicants]
£24,537 NHS Grampian. (2018-2019). Achieving Self-Directed Integrated Cancer Aftercare (ASICA): developing digitally supported cancer aftercare to achieve high quality, equitable care for diverse populations. Murchie, P [PI], Maclennan SJ et al. [co-applicants]
£9,805 GCRF-IPPF3 (2018). Increasing participation in breast cancer screening and treatment within Manipal, India (CANTREAT). MacLennan, SJ., & Poobalan, A.
£5,341,011 EU IMI (2018-2023). Prostate Cancer Diagnosis and TreatmeNt Enhancement through the Power of Big Data in EuRope – PIONEER (2018 – 2023). PIONEER Consortium – MacLennan SJ – lead applicant for University of Aberdeen group.
£2,271,424 NIHR HTA (2018-2023) Randomised Controlled Trial CompAring THE Clinical And CosT- Effectiveness Of Various Washout Policies Versus No Washout Policy In Preventing Catheter Associated Complications In Adults Living With Long-Term Catheters (CATHETER II Study). Abdel-Fattah, M. [PI], Maclennan SJ et al. [co-applicants]
£175,000 NHS Grampian Endowments (via UCAN) (2018-2021). EVOLVE: Giving Patients a Meaningful Voice in the Design and Delivery of Care. S J MacLennan
£50,000 European Association of Urology (2017-2018). Review of Renal Cell Carcinoma: Muscle-invasive & Metastatic Bladder Cancer (Lymph node Dissection) & Penile Cancer. N’Dow, J., & MacLennan, S.J.
£123,357 UCAN (2017- 2019). Meeting the Needs of Patients: Developing Core outcome Sets for Urological Cancer. MacLennan, S.J.
€1,070 EU COST action STSM-IS1211-35195 (2016). Cancer and Work Participation: Research visit to Dr Angela de Boer, Coronel Institute for Occupational Health, Academic Medical Centre, Amsterdam. MacLennan, SJ.
£11,548 NHS Grampian Endowments Award (2015 – 2016). Understanding the hard choices made by working women with breast cancer between treatment compliance and working on: clinical vs economic survival. Improving the Role of the NHS in Grampian. MacLennan SJ., Cox T., N’Dow, J & Heys, S.
£750 Santander Mobility Award (2015), University of Aberdeen. Developing the METIS Collaboration: Cancer Survivorship, Work & Working Life. MacLennan SJ.
£29,500 ESRC (2014 – 2017) Social Science Perspectives on the Working Lives of Those with Cancer: Psychosocial, Organisational and Economic Perspectives. Cox, T., MacLennan, SJ., Hassard, J., & Brown, H.
£25,000 Birkbeck University of London (2014) The Metis Collaboration: Cancer Survivorship, Work & Working Life. MacLennan, SJ.
£109,646 Macmillan Cancer Support (2013 – 2015) Cancer Survivorship: The Patient Journey, Work & Working Life. MacLennan, SJ., Cox, T., & N’Dow, J.
£1,426,755 Health Technology Assessment Programme (HTA) (2013 – 2017)Therapeutic Interventions for Stones of the Ureter (TISU): a multicentre randomised controlled trial of extracorporeal shockwave lithotripsy, as first treatment option, compared with direct progression to ureteroscopic retrieval, for ureteric stones. (McClinton,S., N’Dow, J., MacLennan, GS., Kilonzo, M., Keeley, F., Anson, K., Clark, C., Pickard, R., Norrie, J., MacLennan, SJ., Thomas, R., Starr, K., Burgess, N., Lam, T., Kurban, L.)
£292,253 Health Technology Assessment Programme (HTA) (2012 – 2015) Ablative therapy for men with localised prostate cancer (Ramsay, C., Pickard, R., Vale, L., Lam, T., Mowatt, G., MacLennan, SJ., Rushton, S., N’Dow, J., Merseburger, A., Shirley, M., & Heidenreich, A. )
£176,903 Cancer Research Aberdeen & North East Scotland (CRANES) (2012 – 2015) Development of core outcomes for surgical management of localised prostate cancer to support decision-making by patients, clinicians and policy makers (Lam, T., N’Dow, J., MacLennan, SJ., Ramsay, C., & Campbell, M)
£24,680 The Prostate Cancer Charity Scotland (2011 – 2012) Support groups for men who have prostate cancer, their families and friends: identifying best practice models (MacLennan, SJ., Skea, Z, N’Dow, J & McCann, S)
£14,042 Scottish Cancer Research Network (2011 – 2012) Information for choice in urological cancer: What people need, prefer and use (MacLennan, SJ., & Skea, Z)
£10,317 Scottish Cancer Research Network (2010 – 2011) Delivering Peer Support Interventions in Urological Cancer (Employing a research nurse) (MacLennan, SJ., & N’Dow, J)
£150,700UCAN (Urological Cancer Charity) (2008 – 2013) Addressing the gaps in evidence for treatment of urological cancers (Employing a systematic reviewer) (N’Dow, J., MacLennan, SJ., & Imamura, M)
£165,975 The Scottish Government – CSO (2009 – 2012) Postdoctoral Training Fellowship in Health Services and Health of Public Research – The Acceptability and Usefulness of a Trial Participation Decision Aid: A Mixed Methods Study of Patients and Clinicians in the UK (Schumm, K., Campbell, M., Ramsay, C., N’Dow, J., Skea, Z., & MacLennan, SJ)
£245,482 UCAN (Urological Cancer Charity) (2009 – 2013) Information needs of those living with urological cancer (MacLennan, SJ)
£147,000 Macmillan in partnership with UCAN (2009 – 2012) Making life better for those living with urological cancer (MacLennan, SJ)
Datasets
-
Health status and associated factors of middle-aged and older adult cancer survivors in India: results from the Longitudinal Ageing Study in India
Background The number of persons who have survived cancer has been increasing in India as elsewhere due to advances in detection and treatment of this disease. However, evidence on the standardised number of cancer survivors, their characteristics and their complex health challenges on a national level does not exist due to data limitations. This study, therefore, examines the profile of cancer survivors and their health status using the recently released Longitudinal Ageing Study in India (LASI) survey data. Methods LASI wave 1 is a cross-sectional nationally representative survey of 65,562 middle and older adults aged 45 and above. We first calculated the socioeconomic, demographic and geographical characteristics of cancer survivors (per 100,000 population). We later estimated the adjusted odds of poor health, sleep problems, depressive symptoms, activities of living limitations (ADL and IADL), and hospitalisation of cancer survivors using multivariable logistic regression analysis. Results According to LASI estimates, there were 2.1 million cancer survivors in India (95% CI 1.8 million to 2.6 million) in 2017–18. Overall, 440 cancer survivors have been identified in this study, with considerable state variations. The number of cancer survivors per 1,00,000 population was relatively more in non-indigenous groups, people with a history of cancer in their families, those who worked earlier but currently not working and those in the richest quintile categories. As compared to those who never had cancer, the cancer survivors are at higher risk of hospitalisation (adjusted odds ratio (aOR) = 2.61 CI 1.86, 3.67), poor self-rated health (aOR = 3.77, CI 2.55, 5.54), depressive symptoms (aOR = 1.53, CI 1.41, 2.05) and sleep problems (aOR = 2.29, CI 1.50, 3.47). They also reported higher ADL (aOR = 1.61, CI 1.11, 2.34) and IADL (aOR = 1.49, CI 1.07, 2.07) limitations. Cancer survivors who had their cancer diagnosis in the past 2 years or a cancer-related treatment in the past 2 years have significantly higher odds of poor health status than middle-aged and older adults without a cancer history. Conclusion Middle-aged and older cancer survivors, particularly those who underwent cancer diagnosis or treatment in the past 2 years, are at a significantly higher risk of experiencing poor self-reported health and other health challenges, suggesting the need for an integrated healthcare approach.- DOI
- 10.6084/m9.figshare.c.6262030.v1
- Publisher
- Figshare
- Links
- Date Made Available
- 23 October 2022
- Related Research Outputs
- Contributors
- Guntupalli, A. M. (Creator), Selvamani, Y. (Creator), Maclennan, S. J. (Creator), Dilip, T. R. (Creator)
-
Data From Effectiveness of de-implementation strategies for low-value prescribing in secondary care: a systematic review
- DOI
- 10.1186/s43058-023-00498-0
- Publisher
- University of Aberdeen
- Links
-
- https://static-content.springer.com/esm/art%3A10.1186%2Fs43058-023-00498-0/MediaObjects/43058_2023_498_MOESM1_ESM.docx
- https://static-content.springer.com/esm/art%3A10.1186%2Fs43058-023-00498-0/MediaObjects/43058_2023_498_MOESM2_ESM.docx
- https://static-content.springer.com/esm/art%3A10.1186%2Fs43058-023-00498-0/MediaObjects/43058_2023_498_MOESM3_ESM.xlsx
- https://static-content.springer.com/esm/art%3A10.1186%2Fs43058-023-00498-0/MediaObjects/43058_2023_498_MOESM4_ESM.xlsx
- https://static-content.springer.com/esm/art%3A10.1186%2Fs43058-023-00498-0/MediaObjects/43058_2023_498_MOESM5_ESM.xlsx
- https://static-content.springer.com/esm/art%3A10.1186%2Fs43058-023-00498-0/MediaObjects/43058_2023_498_MOESM6_ESM.xlsx
- Date Made Available
- 18 December 2023
- Related Research Outputs
- Contributors
- Dunsmore, J. (Creator), Duncan, E. (Creator), MacLennan, S. (Creator), N'Dow, J. (Creator), MacLennan, S. (Creator)
-
Data From Effectiveness of de-implementation strategies for low-value prescribing in secondary care: a systematic review
- DOI
- 10.1186/s43058-023-00498-0
- Publisher
- University of Aberdeen
- Links
-
- https://static-content.springer.com/esm/art%3A10.1186%2Fs43058-023-00498-0/MediaObjects/43058_2023_498_MOESM1_ESM.docx
- https://static-content.springer.com/esm/art%3A10.1186%2Fs43058-023-00498-0/MediaObjects/43058_2023_498_MOESM2_ESM.docx
- https://static-content.springer.com/esm/art%3A10.1186%2Fs43058-023-00498-0/MediaObjects/43058_2023_498_MOESM3_ESM.xlsx
- https://static-content.springer.com/esm/art%3A10.1186%2Fs43058-023-00498-0/MediaObjects/43058_2023_498_MOESM4_ESM.xlsx
- https://static-content.springer.com/esm/art%3A10.1186%2Fs43058-023-00498-0/MediaObjects/43058_2023_498_MOESM5_ESM.xlsx
- https://static-content.springer.com/esm/art%3A10.1186%2Fs43058-023-00498-0/MediaObjects/43058_2023_498_MOESM6_ESM.xlsx
- Date Made Available
- 18 December 2023
- Related Research Outputs
- Contributors
- Dunsmore, J. (Creator), Duncan, E. (Creator), MacLennan, S. (Creator), N'Dow, J. (Creator), MacLennan, S. (Creator)
-
Data From Influences on androgen deprivation therapy prescribing before surgery in high-risk prostate cancer
- DOI
- 10.1002/bco2.411
- Publisher
- University of Aberdeen
- Links
- Date Made Available
- 01 July 2024
- Related Research Outputs
- Contributors
- Dunsmore, J. (Creator), Duncan, E. (Creator), MacLennan, S. (Creator), N'Dow, J. (Creator), Cornford, P. (Creator), Esperto, F. (Creator), Pavan, N. (Creator), Ribal, M. J. (Creator), Roobol, M. J. (Creator), Skolarus, T. A. (Creator), MacLennan, S. (Creator)
-
Data From Influences on androgen deprivation therapy prescribing before surgery in high-risk prostate cancer
- DOI
- 10.1002/bco2.411
- Publisher
- University of Aberdeen
- Links
- Date Made Available
- 01 July 2024
- Related Research Outputs
- Contributors
- Dunsmore, J. (Creator), Duncan, E. (Creator), MacLennan, S. (Creator), N'Dow, J. (Creator), Cornford, P. (Creator), Esperto, F. (Creator), Pavan, N. (Creator), Ribal, M. J. (Creator), Roobol, M. J. (Creator), Skolarus, T. A. (Creator), MacLennan, S. (Creator)
-
Effectiveness of de-implementation strategies for low-value prescribing in secondary care: a systematic review - Additional file 6
Additional file 6 of Effectiveness of de-implementation strategies for low-value prescribing in secondary care: a systematic review Additional file 6. Table of Reported consequences in included studies.- DOI
- 10.6084/m9.figshare.26618200
- Publisher
- Figshare
- Links
- Date Made Available
- 07 March 2026
- Contributors
- Dunsmore, J. (Creator), Duncan, E. (Creator), MacLennan, S. (Creator), N'Dow, J. (Creator), MacLennan, S. (Creator), St Clair Gibson, A. (Other)
-
Effectiveness of de-implementation strategies for low-value prescribing in secondary care: a systematic review - Additional file 6
Additional file 6 of Effectiveness of de-implementation strategies for low-value prescribing in secondary care: a systematic review Additional file 6. Table of Reported consequences in included studies.- DOI
- 10.6084/m9.figshare.26618200
- Publisher
- Figshare
- Links
- Date Made Available
- 07 March 2026
- Contributors
- Dunsmore, J. (Creator), Duncan, E. (Creator), MacLennan, S. (Creator), N'Dow, J. (Creator), MacLennan, S. (Creator), St Clair Gibson, A. (Other)
-
Effectiveness of de-implementation strategies for low-value prescribing in secondary care: a systematic review - Additional file 3
Additional file 3 of Effectiveness of de-implementation strategies for low-value prescribing in secondary care: a systematic review Additional file 3. Table of Outcomes and effects of the intervention.- DOI
- 10.6084/m9.figshare.26618191
- Publisher
- Figshare
- Links
- Date Made Available
- 07 March 2026
- Contributors
- Dunsmore, J. (Creator), Duncan, E. (Creator), MacLennan, S. (Creator), N'Dow, J. (Creator), MacLennan, S. (Creator), St Clair Gibson, A. (Other)
-
Effectiveness of de-implementation strategies for low-value prescribing in secondary care: a systematic review - Additional file 3
Additional file 3 of Effectiveness of de-implementation strategies for low-value prescribing in secondary care: a systematic review Additional file 3. Table of Outcomes and effects of the intervention.- DOI
- 10.6084/m9.figshare.26618191
- Publisher
- Figshare
- Links
- Date Made Available
- 07 March 2026
- Contributors
- Dunsmore, J. (Creator), Duncan, E. (Creator), MacLennan, S. (Creator), N'Dow, J. (Creator), MacLennan, S. (Creator), St Clair Gibson, A. (Other)
-
Specifying behavioural and strategy components of de-implementation efforts targeting low-value prescribing practices in secondary health care - Additional file 1
Additional file 1 of Specifying behavioural and strategy components of de-implementation efforts targeting low-value prescribing practices in secondary health care Additional file 1. Identification of the behavioural elements as coded to the AACTT framework domians for control groups.- DOI
- 10.6084/m9.figshare.26517854.v1
- Publisher
- Figshare
- Links
- Date Made Available
- 07 March 2026
- Contributors
- Dunsmore, J. (Creator), Duncan, E. (Creator), MacLennan, S. (Creator), N'Dow, J. (Creator), MacLennan, S. (Creator), St Clair Gibson, A. (Other)
-
Specifying behavioural and strategy components of de-implementation efforts targeting low-value prescribing practices in secondary health care - Additional file 1
Additional file 1 of Specifying behavioural and strategy components of de-implementation efforts targeting low-value prescribing practices in secondary health care Additional file 1. Identification of the behavioural elements as coded to the AACTT framework domians for control groups.- DOI
- 10.6084/m9.figshare.26517854.v1
- Publisher
- Figshare
- Links
- Date Made Available
- 07 March 2026
- Contributors
- Dunsmore, J. (Creator), Duncan, E. (Creator), MacLennan, S. (Creator), N'Dow, J. (Creator), MacLennan, S. (Creator), St Clair Gibson, A. (Other)
-
Effectiveness of de-implementation strategies for low-value prescribing in secondary care: a systematic review - Additional file 4
Additional file 4 of Effectiveness of de-implementation strategies for low-value prescribing in secondary care: a systematic review Additional file 4. Table of Barriers to de-implementation categorised to the TDF Domains.- DOI
- 10.6084/m9.figshare.26618194.v1
- Publisher
- Figshare
- Links
- Date Made Available
- 07 March 2026
- Contributors
- Dunsmore, J. (Creator), Duncan, E. (Creator), MacLennan, S. (Creator), N'Dow, J. (Creator), MacLennan, S. (Creator), St Clair Gibson, A. (Other)
-
Effectiveness of de-implementation strategies for low-value prescribing in secondary care: a systematic review - Additional file 4
Additional file 4 of Effectiveness of de-implementation strategies for low-value prescribing in secondary care: a systematic review Additional file 4. Table of Barriers to de-implementation categorised to the TDF Domains.- DOI
- 10.6084/m9.figshare.26618194.v1
- Publisher
- Figshare
- Links
- Date Made Available
- 07 March 2026
- Contributors
- Dunsmore, J. (Creator), Duncan, E. (Creator), MacLennan, S. (Creator), N'Dow, J. (Creator), MacLennan, S. (Creator), St Clair Gibson, A. (Other)
-
Effectiveness of de-implementation strategies for low-value prescribing in secondary care: a systematic review - Additional file 5
Additional file 5 of Effectiveness of de-implementation strategies for low-value prescribing in secondary care: a systematic review Additional file 5. Table of Facilitators to de-implementation categorised to the TDF Domains.- DOI
- 10.6084/m9.figshare.26618197.v1
- Publisher
- Figshare
- Links
- Date Made Available
- 07 March 2026
- Contributors
- Dunsmore, J. (Creator), Duncan, E. (Creator), MacLennan, S. (Creator), N'Dow, J. (Creator), MacLennan, S. (Creator), St Clair Gibson, A. (Other)
-
Effectiveness of de-implementation strategies for low-value prescribing in secondary care: a systematic review - Additional file 5
Additional file 5 of Effectiveness of de-implementation strategies for low-value prescribing in secondary care: a systematic review Additional file 5. Table of Facilitators to de-implementation categorised to the TDF Domains.- DOI
- 10.6084/m9.figshare.26618197.v1
- Publisher
- Figshare
- Links
- Date Made Available
- 07 March 2026
- Contributors
- Dunsmore, J. (Creator), Duncan, E. (Creator), MacLennan, S. (Creator), N'Dow, J. (Creator), MacLennan, S. (Creator), St Clair Gibson, A. (Other)
-
Effectiveness of de-implementation strategies for low-value prescribing in secondary care: a systematic review
Abstract Background/aims Considerable efforts have been made to improve guideline adherence in healthcare through de-implementation, such as decreasing the prescription of inappropriate medicines. However, we have limited knowledge about the effectiveness, barriers, facilitators and consequences of de-implementation strategies targeting inappropriate medication prescribing in secondary care settings. This review was conducted to understand these factors to contribute to better replication and optimisation of future de-implementation efforts to reduce low-value care. Methods A systematic review of randomised control trials was conducted. Papers were identified through CINAHL, EMBASE, MEDLINE and Cochrane register of controlled trials to February 2021. Eligible studies were randomised control trials evaluating behavioural strategies to de-implement inappropriate prescribing in secondary healthcare. Risk of bias was assessed using the Cochrane Risk of Bias tool. Intervention characteristics, effectiveness, barriers, facilitators and consequences were identified in the study text and tabulated. Results Eleven studies were included, of which seven were reported as effectively de-implementing low-value prescribing. Included studies were judged to be mainly at low to moderate risk for selection biases and generally high risk for performance and reporting biases. The majority of these strategies were clinical decision support at the ‘point of care’. Clinical decision support tools were the most common and effective. They were found to be a low-cost and simple strategy. However, barriers such as clinician’s reluctance to accept recommendations, or the clinical setting were potential barriers to their success. Educational strategies were the second most reported intervention type however the utility of educational strategies for de-implementation remains varied. Multiple barriers and facilitators relating to the environmental context, resources and knowledge were identified across studies as potentially influencing de-implementation. Various consequences were identified; however, few measured the impact of de-implementation on usual appropriate practice. Conclusion This review offers insight into the intervention strategies, potential barriers, facilitators and consequences that may affect the de-implementation of low-value prescribing in secondary care. Identification of these key features helps understand how and why these strategies are effective and the wider (desirable or undesirable) impact of de-implementation. These findings can contribute to the successful replication or optimisation of strategies used to de-implement low-value prescribing practices in future. Trial registration The review protocol was registered at PROSPERO (ID: CRD42021243944).- DOI
- 10.6084/m9.figshare.c.6841186.v1
- Publisher
- Figshare
- Links
- Date Made Available
- 07 March 2026
- Related Research Outputs
- Contributors
- Dunsmore, J. (Creator), Duncan, E. (Creator), MacLennan, S. (Creator), N’Dow, J. (Creator), MacLennan, S. (Creator)
-
Effectiveness of de-implementation strategies for low-value prescribing in secondary care: a systematic review
Abstract Background/aims Considerable efforts have been made to improve guideline adherence in healthcare through de-implementation, such as decreasing the prescription of inappropriate medicines. However, we have limited knowledge about the effectiveness, barriers, facilitators and consequences of de-implementation strategies targeting inappropriate medication prescribing in secondary care settings. This review was conducted to understand these factors to contribute to better replication and optimisation of future de-implementation efforts to reduce low-value care. Methods A systematic review of randomised control trials was conducted. Papers were identified through CINAHL, EMBASE, MEDLINE and Cochrane register of controlled trials to February 2021. Eligible studies were randomised control trials evaluating behavioural strategies to de-implement inappropriate prescribing in secondary healthcare. Risk of bias was assessed using the Cochrane Risk of Bias tool. Intervention characteristics, effectiveness, barriers, facilitators and consequences were identified in the study text and tabulated. Results Eleven studies were included, of which seven were reported as effectively de-implementing low-value prescribing. Included studies were judged to be mainly at low to moderate risk for selection biases and generally high risk for performance and reporting biases. The majority of these strategies were clinical decision support at the ‘point of care’. Clinical decision support tools were the most common and effective. They were found to be a low-cost and simple strategy. However, barriers such as clinician’s reluctance to accept recommendations, or the clinical setting were potential barriers to their success. Educational strategies were the second most reported intervention type however the utility of educational strategies for de-implementation remains varied. Multiple barriers and facilitators relating to the environmental context, resources and knowledge were identified across studies as potentially influencing de-implementation. Various consequences were identified; however, few measured the impact of de-implementation on usual appropriate practice. Conclusion This review offers insight into the intervention strategies, potential barriers, facilitators and consequences that may affect the de-implementation of low-value prescribing in secondary care. Identification of these key features helps understand how and why these strategies are effective and the wider (desirable or undesirable) impact of de-implementation. These findings can contribute to the successful replication or optimisation of strategies used to de-implement low-value prescribing practices in future. Trial registration The review protocol was registered at PROSPERO (ID: CRD42021243944).- DOI
- 10.6084/m9.figshare.c.6841186.v1
- Publisher
- Figshare
- Links
- Date Made Available
- 07 March 2026
- Related Research Outputs
- Contributors
- Dunsmore, J. (Creator), Duncan, E. (Creator), MacLennan, S. (Creator), N’Dow, J. (Creator), MacLennan, S. (Creator)
-
Specifying behavioural and strategy components of de-implementation efforts targeting low-value prescribing practices in secondary health care
Data from: Specifying behavioural and strategy components of de-implementation efforts targeting low-value prescribing practices in secondary health care Background /Aims De-implementation, including the removal or reduction of unnecessary or inappropriate prescribing, is crucial to ensure patients receive appropriate evidence-based health care. The utilization of de-implementation efforts is contingent on the quality of strategy reporting. To further understand effective ways to de-implement medical practices, specification of behavioural targets and components of de-implementation strategies are required. This paper aims to critically analyse how well the behavioural targets and strategy components, in studies that focused on de-implementing unnecessary or inappropriate prescribing in secondary healthcare settings, were reported. Methods A supplementary analysis of studies included in a recently published review of de-implementation studies was conducted. Article text was coded verbatim to two established specification frameworks. Behavioural components were coded deductively to the five elements of the Action, Actor, Context, Target, Time (AACTT) framework. Strategy components were mapped to the nine elements of the Proctor’s ‘measuring implementation strategies’ framework. Results The behavioural components of low-value prescribing, as coded to the AACTT framework, were generally specified well. However, the Actor and Time components were often vague or not well reported. Specification of strategy components, as coded to the Proctor framework, were less well reported. Proctor’s Actor, Action target: specifying targets, Dose and Justification elements were not well reported or varied in the amount of detail offered. We also offer suggestions of additional specifications to make, such as the ‘interactions’ participants have with a strategy. Conclusion Specification of behavioural targets and components of de-implementation strategies for prescribing practices can be accommodated by the AACTT and Proctor frameworks when used in conjunction. These essential details are required to understand, replicate and successfully de-implement unnecessary or inappropriate prescribing. In general, standardisation in the reporting quality of these components is required to replicate any de-implementation efforts. Trial registration Not registered.- DOI
- 10.6084/m9.figshare.c.7392878.v1
- Publisher
- Figshare
- Links
- Date Made Available
- 07 March 2026
- Related Research Outputs
- Contributors
- Dunsmore, J. (Creator), Duncan, E. (Creator), MacLennan, S. (Creator), N'Dow, J. (Creator), MacLennan, S. (Creator)
-
Specifying behavioural and strategy components of de-implementation efforts targeting low-value prescribing practices in secondary health care
Data from: Specifying behavioural and strategy components of de-implementation efforts targeting low-value prescribing practices in secondary health care Background /Aims De-implementation, including the removal or reduction of unnecessary or inappropriate prescribing, is crucial to ensure patients receive appropriate evidence-based health care. The utilization of de-implementation efforts is contingent on the quality of strategy reporting. To further understand effective ways to de-implement medical practices, specification of behavioural targets and components of de-implementation strategies are required. This paper aims to critically analyse how well the behavioural targets and strategy components, in studies that focused on de-implementing unnecessary or inappropriate prescribing in secondary healthcare settings, were reported. Methods A supplementary analysis of studies included in a recently published review of de-implementation studies was conducted. Article text was coded verbatim to two established specification frameworks. Behavioural components were coded deductively to the five elements of the Action, Actor, Context, Target, Time (AACTT) framework. Strategy components were mapped to the nine elements of the Proctor’s ‘measuring implementation strategies’ framework. Results The behavioural components of low-value prescribing, as coded to the AACTT framework, were generally specified well. However, the Actor and Time components were often vague or not well reported. Specification of strategy components, as coded to the Proctor framework, were less well reported. Proctor’s Actor, Action target: specifying targets, Dose and Justification elements were not well reported or varied in the amount of detail offered. We also offer suggestions of additional specifications to make, such as the ‘interactions’ participants have with a strategy. Conclusion Specification of behavioural targets and components of de-implementation strategies for prescribing practices can be accommodated by the AACTT and Proctor frameworks when used in conjunction. These essential details are required to understand, replicate and successfully de-implement unnecessary or inappropriate prescribing. In general, standardisation in the reporting quality of these components is required to replicate any de-implementation efforts. Trial registration Not registered.- DOI
- 10.6084/m9.figshare.c.7392878.v1
- Publisher
- Figshare
- Links
- Date Made Available
- 07 March 2026
- Related Research Outputs
- Contributors
- Dunsmore, J. (Creator), Duncan, E. (Creator), MacLennan, S. (Creator), N'Dow, J. (Creator), MacLennan, S. (Creator)
-
Data from: Effectiveness of de-implementation strategies for low-value prescribing in secondary care: a systematic review
Abstract Background/aims Considerable efforts have been made to improve guideline adherence in healthcare through de-implementation, such as decreasing the prescription of inappropriate medicines. However, we have limited knowledge about the effectiveness, barriers, facilitators and consequences of de-implementation strategies targeting inappropriate medication prescribing in secondary care settings. This review was conducted to understand these factors to contribute to better replication and optimisation of future de-implementation efforts to reduce low-value care. Methods A systematic review of randomised control trials was conducted. Papers were identified through CINAHL, EMBASE, MEDLINE and Cochrane register of controlled trials to February 2021. Eligible studies were randomised control trials evaluating behavioural strategies to de-implement inappropriate prescribing in secondary healthcare. Risk of bias was assessed using the Cochrane Risk of Bias tool. Intervention characteristics, effectiveness, barriers, facilitators and consequences were identified in the study text and tabulated. Results Eleven studies were included, of which seven were reported as effectively de-implementing low-value prescribing. Included studies were judged to be mainly at low to moderate risk for selection biases and generally high risk for performance and reporting biases. The majority of these strategies were clinical decision support at the ‘point of care’. Clinical decision support tools were the most common and effective. They were found to be a low-cost and simple strategy. However, barriers such as clinician’s reluctance to accept recommendations, or the clinical setting were potential barriers to their success. Educational strategies were the second most reported intervention type however the utility of educational strategies for de-implementation remains varied. Multiple barriers and facilitators relating to the environmental context, resources and knowledge were identified across studies as potentially influencing de-implementation. Various consequences were identified; however, few measured the impact of de-implementation on usual appropriate practice. Conclusion This review offers insight into the intervention strategies, potential barriers, facilitators and consequences that may affect the de-implementation of low-value prescribing in secondary care. Identification of these key features helps understand how and why these strategies are effective and the wider (desirable or undesirable) impact of de-implementation. These findings can contribute to the successful replication or optimisation of strategies used to de-implement low-value prescribing practices in future. Trial registration The review protocol was registered at PROSPERO (ID: CRD42021243944).- DOI
- 10.6084/m9.figshare.c.6841186
- Publisher
- Figshare
- Links
- Date Made Available
- 07 March 2026
- Related Research Outputs
- Contributors
- Dunsmore, J. (Creator), Duncan, E. (Creator), MacLennan, S. (Creator), N'Dow, J. (Creator), MacLennan, S. (Creator)
-
Data from: Effectiveness of de-implementation strategies for low-value prescribing in secondary care: a systematic review
Abstract Background/aims Considerable efforts have been made to improve guideline adherence in healthcare through de-implementation, such as decreasing the prescription of inappropriate medicines. However, we have limited knowledge about the effectiveness, barriers, facilitators and consequences of de-implementation strategies targeting inappropriate medication prescribing in secondary care settings. This review was conducted to understand these factors to contribute to better replication and optimisation of future de-implementation efforts to reduce low-value care. Methods A systematic review of randomised control trials was conducted. Papers were identified through CINAHL, EMBASE, MEDLINE and Cochrane register of controlled trials to February 2021. Eligible studies were randomised control trials evaluating behavioural strategies to de-implement inappropriate prescribing in secondary healthcare. Risk of bias was assessed using the Cochrane Risk of Bias tool. Intervention characteristics, effectiveness, barriers, facilitators and consequences were identified in the study text and tabulated. Results Eleven studies were included, of which seven were reported as effectively de-implementing low-value prescribing. Included studies were judged to be mainly at low to moderate risk for selection biases and generally high risk for performance and reporting biases. The majority of these strategies were clinical decision support at the ‘point of care’. Clinical decision support tools were the most common and effective. They were found to be a low-cost and simple strategy. However, barriers such as clinician’s reluctance to accept recommendations, or the clinical setting were potential barriers to their success. Educational strategies were the second most reported intervention type however the utility of educational strategies for de-implementation remains varied. Multiple barriers and facilitators relating to the environmental context, resources and knowledge were identified across studies as potentially influencing de-implementation. Various consequences were identified; however, few measured the impact of de-implementation on usual appropriate practice. Conclusion This review offers insight into the intervention strategies, potential barriers, facilitators and consequences that may affect the de-implementation of low-value prescribing in secondary care. Identification of these key features helps understand how and why these strategies are effective and the wider (desirable or undesirable) impact of de-implementation. These findings can contribute to the successful replication or optimisation of strategies used to de-implement low-value prescribing practices in future. Trial registration The review protocol was registered at PROSPERO (ID: CRD42021243944).- DOI
- 10.6084/m9.figshare.c.6841186
- Publisher
- Figshare
- Links
- Date Made Available
- 07 March 2026
- Related Research Outputs
- Contributors
- Dunsmore, J. (Creator), Duncan, E. (Creator), MacLennan, S. (Creator), N'Dow, J. (Creator), MacLennan, S. (Creator)
- Teaching
-
- Publications
-
Page 2 of 3 Results 51 to 100 of 139
Work-related Factors in the etiology of Symptoms of Post-traumatic Stress among First Responders: The Brazilian Firefighters Longitudinal Health Study (FLoHS)
Cadernos de Saúde Pública/Reports in Public Health, vol. 37, no. 9, e00135920Contributions to Journals: ArticlesEVALUATING PATIENT-REPORTED OUTCOMES MEASURES (PROMS) FOR LOCALISED PROSTATE CANCER: A SYSTEMATIC REVIEW USING THE CONSENSUS-BASED STANDARD FOR THE SELECTION OF HEALTH MEASUREMENT INSTRUMENTS (COSMIN) METHODOLOGY
Journal of Urology, vol. 206, no. S3, pp. E212-E212Contributions to Journals: Abstracts- [ONLINE] DOI: https://doi.org/10.1097/JU.0000000000001989.07
What influences adherence to guidance for post-operative instillation of intravesical chemotherapy to bladder cancer patients?
BJU International, vol. 128, no. 2, pp. 225-235Contributions to Journals: ArticlesShockwave Lithotripsy Versus Ureteroscopic Treatment as Therapeutic Interventions for Stones of the Ureter (TISU): A Multicentre Randomised Controlled Non-inferiority Trial
European Urology, vol. 80, no. 1, pp. 46-54Contributions to Journals: ArticlesEvaluating Patient-Reported Outcomes Measures (PROMs) for localised prostate cancer: A systematic review using the Consensus-based Standard for the selection of health Measurement Instruments (COSMIN) methodology
EAU21 Virtual Congress, pp. S430-S431Contributions to Journals: Abstracts- [ONLINE] DOI: https://doi.org/10.1016/S0302-2838(21)00686-2
TISU (Therepeutic Intervention for Stones in the Ureter): ESWL versus Ureteroscopy, a multicentre RCT
European Urology, vol. 79, no. S1, pp. S390-S390Contributions to Journals: Abstracts- [ONLINE] DOI: https://doi.org/10.1016/S0302-2838(21)00660-6
Diagnostic and prognostic factors in patients with prostate cancer: a systematic review protocol
BMJ Open, vol. 11, no. 2, e040531Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1136/bmjopen-2020-040531
- [OPEN ACCESS] http://aura.abdn.ac.uk/bitstreams/380ed043-085a-4f21-9cb7-048811cc7529/download
- [ONLINE] View publication in Scopus
Providing a Framework for Meaningful Patient Involvement in Clinical Practice Guideline Development and Implementation
European Urology Focus, vol. 7, no. 5, pp. 947-950Contributions to Journals: ArticlesThe Key Role of Patient Involvement in the Development of Core Outcome Sets in Prostate Cancer
European Urology FocusContributions to Journals: ArticlesIncreasing Engagement for Breast Cancer Screening and Treatment: The "ICANTREAT" Community of Expertise Initiative
Asian Pacific Journal of Cancer Prevention, vol. 21, no. 12, pp. 3655-3659Contributions to Journals: ArticlesPIONEER’s systematic review of outcomes in RCTs of men with non-metastatic castration resistant prostate cancer: Is there a need for a core outcome set?
35th Annual EAU CongressContributions to Conferences: AbstractsInitial steps of an online search tool development for diagnostic and prognostic factors in prostate cancer
12th European Multidisciplinary Congress on Urological Cancers 2020Contributions to Conferences: AbstractsPIONEER’s operational definitions:: Harmonising clinical characteristics and phenotypes data of prostate cancer patients
12th European Multidisciplinary Congress on Urological Cancers 2020Contributions to Conferences: AbstractsIdentifying the most appropriate instrument for patient reported outcome measure (PROMS) in patients with clinically localized prostate cancer
12th European Multidisciplinary Congress on Urological Cancers 2020Contributions to Conferences: AbstractsPIONEER consensus on clinician reported outcome measurements
12th European Multidisciplinary Congress on Urological Cancers 2020Contributions to Conferences: AbstractsEVOLVE: a framework for meaningful patient involvement in clinical practice guideline development and implementation
Cochrane Database of Systematic Reviews, vol. 9 Suppl 1, pp. 26Contributions to Journals: AbstractsNo turning back’ Psycho-oncology in the time of COVID-19: Insights from a survey of UK professionals
Psycho-Oncology, vol. 29, no. 9, pp. 1430-1435Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1002/pon.5486
- [OPEN ACCESS] http://aura.abdn.ac.uk/bitstreams/d66ef77d-5430-41cc-9539-42da0b9dd2e1/download
- [ONLINE] View publication in Scopus
Author Correction: Introducing PIONEER: a project to harness big data in prostate cancer research (Nature Reviews Urology, (2020), 17, 6, (351-362), 10.1038/s41585-020-0324-x)
Nature Reviews Urology, vol. 17, pp. 482Contributions to Journals: Comments and Debates- [ONLINE] DOI: https://doi.org/10.1038/s41585-020-0355-3
- [ONLINE] View publication in Scopus
- [ONLINE] Springer Nature SharedIt link to author correction.
Benefits of an embedded qualitative study within clinical trials: Patient values and preferences
35th Annual EAU CongressContributions to Conferences: AbstractsIntroducing PIONEER: a project to harness big data in prostate cancer research
Nature reviews. Urology, vol. 17, pp. 351-362Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1038/s41585-020-0355-3
- [OPEN ACCESS] http://aura.abdn.ac.uk/bitstreams/55bac675-79ac-49c2-88db-3cb46ce1500a/download
- [ONLINE] View publication in Scopus
PIONEER’s systematic review of outcomes reported in effectiveness trials for interventions in locally advanced prostate cancer
11th European Multidisciplinary Congress on Urological CancersContributions to Conferences: AbstractsPIONEER’s update and integration of a localised prostate cancer core outcome set for effectiveness trials and a standard set for clinical practice
11th European Multidisciplinary Congress on Urological CancersContributions to Conferences: AbstractsEVOLVE: Designing a model of meaningful patient involvement in guideline development
European Urology Supplements, vol. 18, no. 1, pp. e2117Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1016/s1569-9056(19)31534-9
TISU: Extracorporeal shockwave lithotripsy, as first treatment option, compared with direct progression to ureteroscopic treatment, for ureteric stones: study protocol for a randomised controlled trial
Trials, vol. 19, no. 1, pp. 1-11Contributions to Journals: ArticlesCare pathways for the management of localised and locally advanced prostate cancer:: Experience of the EAU guidelines office
33rd Annual EAU Congress 2018, pp. PT075Contributions to Conferences: PostersCare pathways for the management of metastatic and castration-resistant prostate cancer in the era of novel therapeutic options:: Experience of the EAU guidelines office
33rd Annual EAU Congress 2018Contributions to Conferences: Posters“Throughout the cancer patient's journey, there ought to be a discussion about work”: The role of GPs in Scotland
Psycho-Oncology, vol. 27, no. 1, pp. 343-346Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1002/pon.4407
- [OPEN ACCESS] http://aura.abdn.ac.uk/bitstreams/8abf23f0-4bc7-464d-b57f-d9ffd2b92cb4/download
The relationship between social support and health-related quality of life in patients with antiphospholipid (hughes) syndrome
Modern Rheumatology , vol. 28, no. 1, pp. 147-155Contributions to Journals: ArticlesA core outcome set for localised prostate cancer effectiveness trials
BJU International, vol. 120, no. 5B, pp. E64-E79Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1111/bju.13854
- [ONLINE] View publication in Scopus
A core outcome set for localised prostate cancer effectiveness trials
BJU International, vol. 120, no. 5B, pp. E64-79Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1111/bju.13854
- [OPEN ACCESS] http://aura.abdn.ac.uk/bitstreams/4e075d50-8d28-40a2-b9fd-b3235a65a2f1/download
Changing current practice in urological cancer care: Providing better information, advice and related support on work engagement
European Journal of Cancer Care, vol. 26, no. 5, e12756Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1111/ecc.12756
- [OPEN ACCESS] http://aura.abdn.ac.uk/bitstreams/f9146484-615c-48e0-827f-303a0732b145/download
Changing Current Practice in Urology: Improving Guideline Development and Implementation Through Stakeholder Engagement
European Urology, vol. 72, no. 2, pp. 161-163Contributions to Journals: EditorialsThe Relationship Between Social Support and Health-Related Quality of Life in Patients with Anti-Phospholipid Syndrome
Annual Meeting of the British-Society-for-Rheumatology, British-Health-Professionals-in-Rheumatology and the British-Society-for-Paediatric-and-Adolescent-Rheumatology (Rheumatology), pp. 110-110Contributions to Journals: AbstractsPatient-centered Care in Maternity Services: A Critical Appraisal and Synthesis of the Literature
Women's Health Issues, vol. 26, no. 1, pp. 100-109Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1016/j.whi.2015.09.003
- [OPEN ACCESS] http://aura.abdn.ac.uk/bitstreams/89569bce-da86-4553-a9fc-3d32ad35eae7/download
- [ONLINE] View publication in Scopus
Exploring the Role of General Practitioners in the Provision of Work-Related Advice, Information and Support to People with Cancer in Scotland: A Grounded Theory Study
NHS Scotland Research Conference 2015Contributions to Conferences: PostersSurgical management for localised penile cancer: [Cochrane Protocol]
Contributions to Journals: Registered ReportsAblative therapy for people with localised prostate cancer: a systematic review and economic evaluation
Health Technology Assessment, vol. 19, no. 49, pp. 1-524Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.3310/hta19490
- [OPEN ACCESS] http://aura.abdn.ac.uk/bitstreams/dc0e1908-42e2-4036-a179-668829243d3a/download
The influences of nursing transformational leadership style on the quality of nurses’ working lives in Taiwan: a cross-sectional quantitative study
BMC Nursing, vol. 14, 33Contributions to Journals: ArticlesWhich type of indwelling urethral catheters should be used for short-term catheterisation in hospitalised adults?: Cochrane Systematic Review of the evidence
21rst Annual Scientific Meeting of the UK Continence SocietyContributions to Conferences: AbstractsSurgical management for localised penile cancer
Cochrane Database of Systematic Reviews, vol. 2015, no. 3, CD011533Contributions to Journals: Articles- [ONLINE] https://doi.org//10.1002/14651858.CD011533
- [ONLINE] DOI: https://doi.org/10.1002/14651858.CD011533
A core outcome set for localised prostate cancer effectiveness trials: protocol for a systematic review of the literature and stakeholder involvement through interviews and a Delphi survey
Trials, vol. 16, 76Contributions to Journals: Literature ReviewsAntiphospholipid (Hughes) syndrome: description of population and health-related quality of life (HRQoL) using the SF-36
Lupus, vol. 24, no. 2, pp. 174-179Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1177/0961203314551809
Cancer Survivorship and Working Life
Contributions to Conferences: Papers- [ONLINE] DOI: https://doi.org/10.1111/j.1099-1611.2014.3697
An exploration of workplace social capital as an antecedent of occupational safety and health climate and outcomes in the Chinese education sector
International Archives of Occupational and Environmental Health , vol. 87, no. 5, pp. 515-526Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1007/s00420-013-0890-9
Cancer survivorship and sustainable working life
Contributions to Conferences: PapersCommunicating good care: A qualitative study of what people with urological cancer value in interactions with health care providers
European Journal of Oncology Nursing, vol. 18, no. 1, pp. 35-40Contributions to Journals: ArticlesTime to act: The challenges of working during and after cancer, initiatives in research and practice
European Journal of Oncology Nursing, vol. 18, no. 1, pp. 1-2Contributions to Journals: EditorialsTypes of indwelling urethral catheters for short-term catheterisation in hospitalised adults
Cochrane Database of Systematic Reviews, no. 9, pp. CD004013Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1002/14651858.CD004013.pub4
- [ONLINE] View publication in Scopus
Determining information for inclusion in a decision-support intervention for clinical trial participation: A modified Delphi approach
Clinical Trials, vol. 10, no. 6, pp. 967-976Contributions to Journals: ArticlesDetermining items for inclusion in a decision support intervention for clinical trial participation: a modified Delphi approach
Contributions to Journals: Conference Articles

