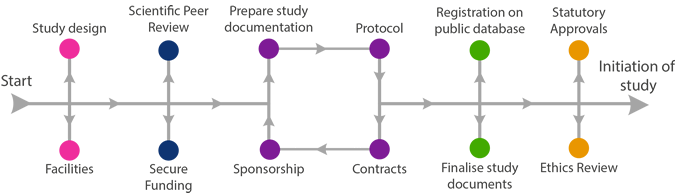

Instructions and advice for the active phase of your research, once all approvals are in place, can be found on the Managing your Research page.
Useful Documents and Links
“Research ethics committees (RECs) act as part of an efficient, accountable and independent Research Ethics Service to protect the dignity, rights, safety and well-being of people who take part in research.” Governance Arrangements for Ethics Committees 3.1.1 2020
North of Scotland Research Ethics Service located within NHS Grampian are part of a UK wide service overseen by the Health Research Authority in the UK and by the Chief Scientist Office in Scotland.
How can we help?
- Advise if your project would require NHS Ethics?
- Link to tools
- Answer questions on the Ethics Process
- Provide links to training and other documentation
- Review documentation before submission via IRAS
Contact details
For more information email gram.nosres@nhs.scot
IRAS - link and further information

