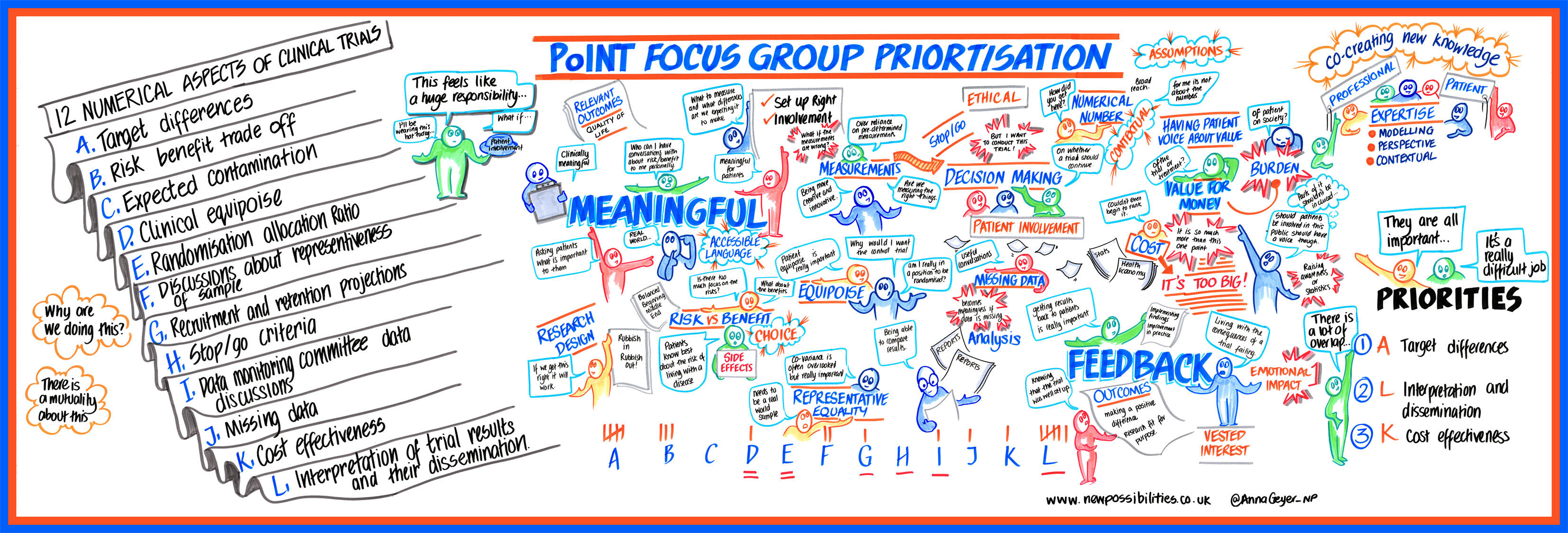
Involving patients and the public in clinical trials has the potential to improve research and its relevance and reduces research waste by improving recruitment. However, to achieve its maximum impact, we need to better understand how to operationalise patient and public involvement (PPI) across all aspects of a trial process. Patients should have the opportunity to be heard in all aspects of clinical trials, including numerical aspects which play an essential role in how trials are designed, analysed and reported. For example, patients could be directly involved in informing the most important analyses questions; or in discussing interpretation of results. Currently, little is known about how patients are involved in numerical aspects of trials.
Our main aim with this project is to ask: what are the important numerical aspects of trials that patients and the public can and want to contribute to?
To achieve this, we are going to:
- search the literature for current practice of involving non-experts in numerical aspects of research.
- ask stakeholders, including patients, to map their experience of taking part in research or trials and reflect on relevant numerical aspects along that process. This will produce a list of potential numerical aspects of clinical trials that patients and the public could be involved in.
- Finally, we will meet with patients, the public, and researchers to discuss these aspects and ensure we know important research questions in the field.
Funder: Wellcome Trust – Institutional Strategic Support Fund at the University of Aberdeen
Project mentors: Marion Campbell, Katie Gillies, Craig Ramsay
Related student projects
Camille Poisson, MSc student in Global Health is currently developing a survey to identify current practice and barriers and facilitators in involving patients in numerical aspects of trials – PoINTS (Public involvement In Numerical aspects of Trials – a survey of current practice, barriers and facilitators to trialists)
Naomi Attard, MSc in Global Health and Management, How do we know if a treatment is good enough? A survey of non-inferiority and equivalence trials
Supervision: Beatriz Goulao, Katie Gillies
To view the results please click here: https://www.youtube.com/watch?v=6bx5T7Kl66Q
Contacts
- Beatriz Goulao; beatriz.goulao@abdn.ac.uk
Status
CompletedPublications
https://bmjopen.bmj.com/content/11/3/e046977
https://trialsjournal.biomedcentral.com/articles/10.1186/s13063-021-05451-x