Outcome of Liver Intervention OR Surveillance in DiSappearing colerectal liver Metastases (LORDS-M Study).
Summary for the public
Every-year in Scotland, approximately 3800 people develop bowel cancer. This spreads to the liver (CRLM) in half of them and is responsible for the majority of deaths. After drug-treatment, around 50% of patients with CRLM, respond positively, and liver lesions disappear on imaging (dCRLM). However, up to 83% of these dCRLM still contain viable cancer. These hidden cancers can regrow aggressively. There are no guidelines on the management of patients with dCRLM.
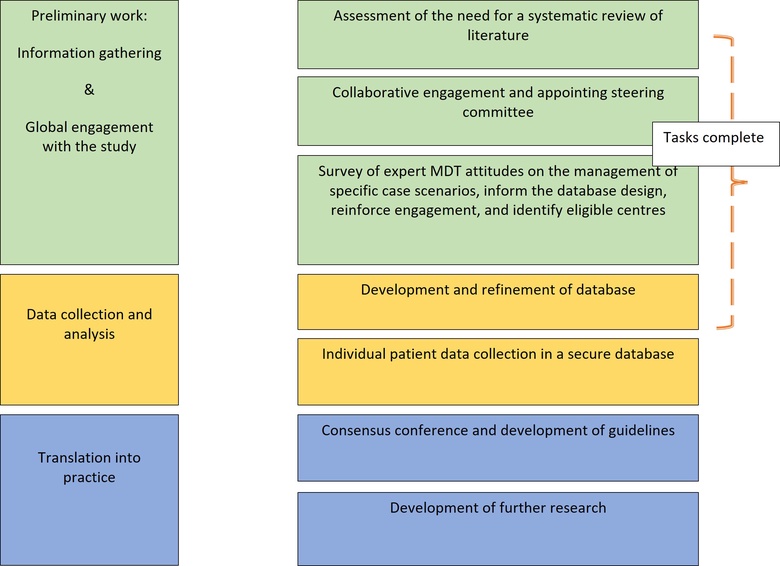
In Scotland, more than two-thirds of the liver surgery is related to CRLM. The high magnitude of this disease remains a major challenge to NHS-Scotland and worldwide. The Scottish team will lead an international group to analyse outcomes of patients’ records collected from Scotland and other countries into a secure database, develop, validate and implement evidence-based guidelines. The group will utilize patients data collected in a secure dedicated database from international centres.
This will inform the clinical practice in Scotland and beyond. In addition, will lead to identification of clinical research priorities in Scotland.
If you have any questions related to the study please email: mohamed.bekheit@nhs.scot
Study Information
- The Clinical Challenge
-
Colorectal cancer (CRC) is amongst the fourth commonest cancer worldwide 1 and is the second leading cause of cancer related death in the UK 2. There are around 42,300 new colorectal cancer cases annually in the UK 3, while Scotland records over 3700 new cases 4. Liver metastases contributed to the death of approximately 50% of these patients 5.
Half of CRC patients develop liver metastasis 6. In Scottish liver centres, two thirds of liver cancer surgery is for colorectal liver metastasis (CRLM). A Recent international survey demonstrated that almost all liver surgeons have experience with disappearing CRLM 7.
Surgical resection remains the standard treatment for CRLM 8. However, it is not always feasible without neoadjuvant chemotherapy 9. Most of the patients with CRLM receive chemotherapy 10 as the condition is often multiple 11, affects both lobes of the liver 12, and approximately half of present with CRC present with high systemic burden of the disease on their first presentation 6 .
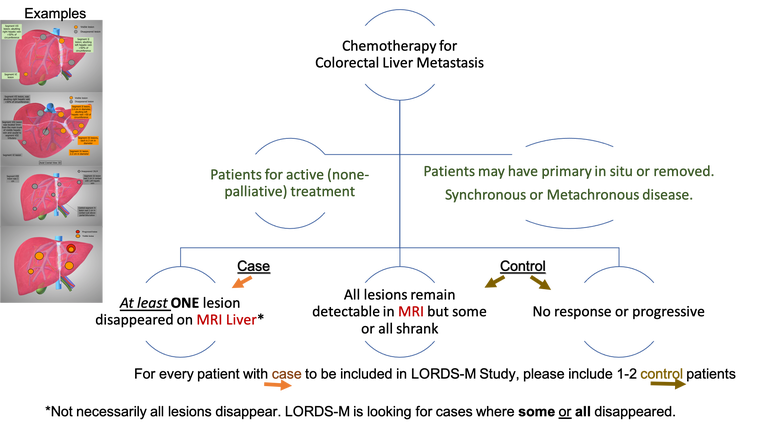
Tumour response to chemotherapy has significantly improved, with up to 50% response rates for initially unresectable liver metastasis, of them 20% proceeding to liver resection with curative intent 13,14. This results in disappearance of some or all of metastatic disease in subsequent investigations, a phenomenon termed “disappearing liver metastases” (dCRLM). Lesions disappear on radiological assessment in approximately half of cases following chemotherapy 15,16. The estimated affected population with dCRLM annually is approximately 360,000 cases globally (see estimated target population in methods). However, only a minority will have a complete pathological response (18% 17 to 25% 18) with the remainder (as high as 83%) having residual active cancer19. This poses a major therapeutic dilemma as most patients with a complete radiological response still have residual disease. Indeed, there is a growing concern that the current observed rate of pathologically viable tumour and rate of early recurrence are alarming 19. Preclinical studies suggested that surgery induces growth of microscopic tumours 20 and in the clinical practice the dropout rates following the first stage hepatectomy for CRLM is a strong support for the potential adverse effect of operating only on visible lesions 21. Therefore, not operating on those lesions may not be justified.
However, the area with viable cancer is substantially difficult to localize intraoperatively and often requires complex techniques and tools, which still have a suboptimal detection rate 22. Furthermore, many of these patients are subject to complications of extending the resection because of residual liver volume limitations 11,23 . Therefore, blind surgery has significant risk of potential morbidity and mortality without guaranteed radicality.
Some surgeons will therefore not offer surgery to these patients, while some advocate observation 24 and others endorse a surgical approach for all patients25.
The majority of CRLM are multiple as previously mentioned and when patients receive chemotherapy, most commonly some of these lesions disappear and some remain detectable. For the purpose of this study and to provide a clear distinction, the situation where some lesion disappeared and some remain detectable will be called mixed disappearing colorectal liver metastasis (mDLMs). Despite the literature addressing the CRLM, high quality studies address neither dCRLM nor mDLMs. A recent systematic review on the management of dCRLM identified low quality studies that require further validation albeit not addressing the fundamental question of this study 26. The fundamental question of this study is whether surgery is equivalent to observation in patients with multiple CRLM in whom some lesions disappeared and others remain detectable on preoperative imaging (mDLMs). Noteworthy the lack of clinical code specific to this condition and the reliance on routine health data did not show yield (communication with public health Scotland).
Publications
https://pubmed.ncbi.nlm.nih.gov/35598157/
References
- International Agency for Research on Cancer (2020). Worldwide Incidence of Colorectal Cancer.
- Research C (2017). Leading Causes of Cancer Related Death in the UK.
- Cancer Research UK (2018). Available at: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/bowel-cancer [Accessed January 20, 2001].
- ISD (2018). Available at: https://www.isdscotland.org/health-topics/cancer/cancer-statistics/ [Accessed January 20, 2020].
- Helling TS, Martin M (2014). Cause of Death from Liver Metastases in Colorectal Cancer. Ann Surg Oncol 21: 501–6. https://doi.org/10.1245/s10434-013-3297-7.
- Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM (2006). Epidemiology and Management of Liver Metastases from Colorectal Cancer. Ann Surg 244: 254–9. https://doi.org/10.1097/01.sla.0000217629.94941.cf.
- Melstrom LG, Warner SG, Wong P, Sun V, Raoof M, Singh G, Chavin KD, Fong Y, et al (2021). Management of Disappearing Colorectal Liver Metastases: An International Survey. Hpb 23: 506–11. https://doi.org/10.1016/j.hpb.2020.10.005.
- Raoof M, Haye S, Ituarte PHG, Fong Y (2019). Liver Resection Improves Survival in Colorectal Cancer Patients: Causal-Effects From Population-Level Instrumental Variable Analysis. Ann Surg 270: 692–700. https://doi.org/10.1097/SLA.0000000000003485.
- Oki E, Ando K, Nakanishi R, Sugiyama M, Nakashima Y, Kubo N, Kudou K, Saeki H, et al (2018). Recent Advances in Treatment for Colorectal Liver Metastasis. Ann Gastroenterol Surg 2: 167–75. https://doi.org/10.1002/ags3.12071.
- Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, et al (2016). ESMO Consensus Guidelines for the Management of Patients with Metastatic Colorectal Cancer. Ann Oncol 27: 1386–422. https://doi.org/10.1093/annonc/mdw235.
- Pawlik TM, Schulick RD, Choti MA (2008). Expanding Criteria for Resectability of Colorectal Liver Metastases. Oncologist 13: 51–64. https://doi.org/10.1634/theoncologist.2007-0142.
- Aloia TA (2006). Solitary Colorectal Liver Metastasis. Arch Surg 141: 460. https://doi.org/10.1001/archsurg.141.5.460.
- Bischof DA, Clary BM, Maithel SK, Pawlik TM (2013). Surgical Management of Disappearing Colorectal Liver Metastases. Br J Surg 100: 1414–20. https://doi.org/10.1002/bjs.9213.
- Kuhlmann K, van Hilst J, Fisher S, Poston G (2016). Management of Disappearing Colorectal Liver Metastases. Eur J Surg Oncol 42: 1798–805. https://doi.org/10.1016/j.ejso.2016.05.005.
- Barimani D, Kauppila JH, Sturesson C, Sparrelid E (2020). Imaging in Disappearing Colorectal Liver Metastases and Their Accuracy: A Systematic Review. World J Surg Oncol 18: 1–9. https://doi.org/10.1186/s12957-020-02037-w.
- Chua TC, Saxena A, Liauw W, Kokandi A, Morris DL (2010). Systematic Review of Randomized and Nonrandomized Trials of the Clinical Response and Outcomes of Neoadjuvant Systemic Chemotherapy for Resectable Colorectal Liver Metastases. Ann Surg Oncol 17: 492–501. https://doi.org/10.1245/s10434-009-0781-1.
- Vujic J, Schöllnast H, Marsoner K, Wienerroither V, Bacher H, Mischinger HJ, Kornprat P (2019). Marking Disappearing Colorectal Liver Metastases after Complete Response to Neoadjuvant Chemotherapy via CT – A Pilot Study. Anticancer Res 39: 3847–54. https://doi.org/10.21873/anticanres.13534.
- Park MJ, Hong N, Han K, Kim MJ, Lee YJ, Park YS, Rha SE, Park S, et al (2017). Use of Imaging to Predict Complete Response of Colorectal Liver Metastases after Chemotherapy: MR Imaging versus CT Imaging. Radiology 284: 423–31. https://doi.org/10.1148/radiol.2017161619.
- Benoist S, Brouquet A, Penna C, Julié C, El Hajjam M, Chagnon S, Mitry E, Rougier P, Nordlinger B (2006). Complete Response of Colorectal Liver Metastases after Chemotherapy: Does It Mean Cure?. J Clin Oncol 24: 3939–45. https://doi.org/10.1200/JCO.2006.05.8727.
- Govaert KM, Jongen JMJ, Kranenburg O, Borel Rinkes IHM (2017). Surgery-Induced Tumor Growth in (Metastatic) Colorectal Cancer. Surg Oncol 26: 535–43. https://doi.org/10.1016/j.suronc.2017.10.004.
- Wicherts DA, Miller R, De Haas RJ, Bitsakou G, Vibert E, Veilhan LA, Azoulay D, Bismuth H, et al (2008). Long-Term Results of Two-Stage Hepatectomy for Irresectable Colorectal Cancer Liver Metastases. Ann Surg 248: 994–1003. https://doi.org/10.1097/SLA.0b013e3181907fd9.
- Pak LM, Gagnière J, Allen PJ, Balachandran VP, D’Angelica MI, DeMatteo RP, Jarnagin WR, Miga MI, et al (2019). Utility of Image Guidance in the Localization of Disappearing Colorectal Liver Metastases. J Gastrointest Surg 23: 760–7. https://doi.org/10.1007/s11605-019-04106-2.
- Truant S, Baillet C, Deshorgue AC, Leteurtre E, Hebbar M, Ernst O, Huglo D, Pruvot F-R (2016). Drop of Total Liver Function in the Interstages of the New Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy Technique. Ann Surg 263: e33–4. https://doi.org/10.1097/SLA.0000000000001603.
- van Vledder MG, de Jong MC, Pawlik TM, Schulick RD, Diaz LA, Choti MA (2010). Disappearing Colorectal Liver Metastases after Chemotherapy: Should We Be Concerned?. J Gastrointest Surg 14: 1691–700. https://doi.org/10.1007/s11605-010-1348-y.
- Tanaka K, Takakura H, Takeda K, Matsuo K, Nagano Y, Endo I (2009). Importance of Complete Pathologic Response to Prehepatectomy Chemotherapy in Treating Colorectal Cancer Metastases. Ann Surg 250: 935–42. https://doi.org/10.1097/SLA.0b013e3181b0c6e4.
- Tsilimigras DI, Ntanasis-Stathopoulos I, Paredes AZ, Moris D, Gavriatopoulou M, Cloyd JM, Pawlik TM (2019). Disappearing Liver Metastases: A Systematic Review of the Current Evidence. Surg Oncol 29: 7–13. https://doi.org/10.1016/j.suronc.2019.02.005.
- The Approach
-
- The information needed
-
Standard information. The study collects standard information on patients who received chemotherapy for colorectal liver metastasis.
- Patient Selection
-
- IRB and Caldicot approval
-
The study has been awarded Caldicott approval through NHS Grampian Caldicott officer and registered as a UK wide study. Colleagues from outside the UK are encouraged to seek local approvals using the available documentations for this purpose.
- Proposed timeline for the data collection phase
-
The study is currently open for data collection. https://redcap.link/lords-m-register follow the link to register and gain access to your database.
Timeline is subject to changes as dictated by the practical circumstances.
- The Study Team
-
Your local team
Having YOU and your team, we can have a better understanding on the best treatment of the disappeared colorectal liver metastasis.
Your collaborative
UK
Patient
Ms
Kim Page
Patient
Ms
Karen O’Malley
France
University Hospital, Lille
Prof
Stephanie Truant
Germany
University of Köln
Prof.Med
Roger Wahba
Italy
San Raffaele Hospital, Milan
Prof
Luca Aldrighetti
Japan
University of Tokyo
Prof
Nobuyuki Takemura
Philippine
National Kidney and Transplant Institute
Dr
Catherine Teh
Russia
University of Moscow
Dr
Ruslan Alikhanov
UK
Grampian
Mr
Mohamed Bekheit
Grampian
Mr
Mudassar Ghazanfar
UK University of Aberdeen
Prof Lesley Anderson Grampian
Prof
Irfan Ahmed
Liverpool
Mr
Robert Jones
Newcastle
Mr
John Hammond
Oxford
Mr
Micheal Silva
Birmingham Mr Bobby Dasari Grampian
Ms
Areeg Noreldin
Grampian
Mr
Amir Abdelhamid
Nottingham Mr Dhanny Gomez USA
Mayo Clinic
Dr
Guido Fiorentini
Ohio State University
Prof
Timothy Pawlik
- Register here to gain access to your database
-
Register your interest here
Please fill out the LORDS-M project registration form to register your interest. Our administrators will then provide you with a REDCap account which can be used to submit your case files.
- Login to submit your case files
-
Login to submit your case files
Please login through the University of Aberdeen REDCap login portal to submit your case files. If you have forgotten your passord, you can reset it here. If you have forgotten your REDCap username please contact digitalresearch@abdn.ac.uk for assistance.
- Your proposed team composition
-
Each site PI is encouraged to choose additional 2 team members. Each site will have around 3 members and if more is required will be considered.
You may want to liaise with colleagues from the following colleagues who may have access to relevant patients records.
- Oncology team (e.g. oncologist, cancer nurse specialist),
- Pathology department (e.g. MDT pathologist for either CRC or HPB)
- Radiology department (e.g. Colorectal cancer or HPB radiologist)
- Colorectal surgical team
- Funding
-
The study is awarded funding for study manager, data curation and analysis.
If you are contributing from the UK, you could claim (into your research fund) reimbursement for time utilised for submission of COMPLETE case records at £30 per hour (approximately £45 per bundle [case + control]). There are additional reimbursement at the same rate for 20% of data quality check from a senior team member different from the members submitting the records.
- Publications
-
Publications
- Authorship
-
All site PIs will be included in named authorship (provided there are complete case records submitted by the centre). All other contributions will be acknowledged in collaborative authorship.
- Previous Publications
-
Nassar A, Cimpean S, Abdelhamid A, Jones RP, Wahba R, Fiorentini G, Aldrighetti L, Teh C, Alikhanov R, Hammond J, Silva M, Abdelmabod A, Truant S, Ferrero A, Sturesson C, Ahmed I, Ghazanfar M, Takemura N, Pawlik TM, Bekheit M. The dilemma of the disappeared colorectal liver metastasis: systematic review of reviews and evidence gap map. BJS Open. 2022 May 2;6(3):zrac051. doi: 10.1093/bjsopen/zrac051. PMID: 35598157; PMCID: PMC9124362.
Ghazanfar et al . The management of dCRLM. International survey. In revision.
