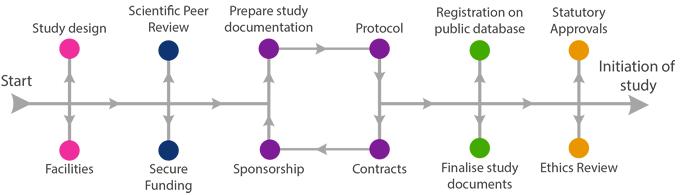
All research governed in the National Health Service or Community Service is governed by the UK Policy Framework for Health and Social Care Research (“RGF”). This Framework requires that all clinical research involving human participants, their organs, tissues or data must have an identified Sponsor who takes responsibility for the initiation, management and financing of a clinical study.
For Studies falling under the Medicines for Human Use (Clinical Trials) Regulations 2004 there are additional legal requirements that the Sponsor must be responsible for.
In the context of research governance the term sponsor has different meaning to the term ‘funder’.
As a Sponsor and/or co-sponsor, the University of Aberdeen or NHS Grampian must ensure that it takes responsibility for the following:
- Assessment of the quality of research proposed, the quality of the research environment within which the research will be undertaken and the expertise of the Chief Investigator and other key staff involved.
- Ensuring arrangements are in place for the research team to access resources and support to deliver the research proposed.
- Ensure agreements which specify the responsibilities for funding, management and monitoring of the research are in place.
- Ensure arrangements are in place to review significant developments as the research proceeds, particularly those which put the safety of individuals at risk, and to approve modifications to the design.
If you are a University of Aberdeen or NHS Grampian employee, or student, and your research involves humans, their tissue and/or data, contact the Research Governance Team as soon as possible to begin the sponsorship process.
The Research Governance Team will:
- Risk assess your project to determine whether sponsorship will be granted
- Advise you on study documentation, such as Protocol, Participant Information Sheet and Informed Consent Forms
- Provide advice on all aspects of running your study
- Discuss any research governance and regulatory issues with you
- Confirm or arrange appropriate Insurance for your study
- Refer the study to the Clinical Study Oversight Group for a full risk assessment if deemed necessary.
Sponsorship MUST be in place, before you can apply to REC, MHRA and R&D for further Approvals.
For more information on what is required to request sponsorship from NHS Grampian and/or University of Aberdeen click here
You should also start preliminary discussions with the Research and Innovation team at this stage to discuss potential contract and/or formal agreement requirements.
Once all the documents have been reviewed by the Sponsor, and any required changes have been made, sponsorship will be confirmed to the Investigator and permission given to apply for further approvals.