PhD, FRSE, FIBiol
Emeritus Professor
- About
-
- Email Address
- i.r.booth@abdn.ac.uk
- Office Address
- School of Medical Sciences Institute of Medical Sciences, Room 6.16 University of Aberdeen Foresterhill Ashgrove Road West Aberdeen AB25 2ZD +44 (0)1224 437396
- School/Department
- School of Medicine, Medical Sciences and Nutrition
Biography
Professor Ian Booth took official retirement from the University of Aberdeen on 31/12/2010, but continues to be closely involved with his former research group and collaborators. The research is funded for research on membrane proteins by both the BBSRC and The Wellcome Trust until 2015. He currently holds a Leverhulme Emeritus Fellowship that supports his joint research with his previous group, now managed by Dr Samantha Miller, and staff at Caltech, Pasadena.
Professor Booth joined the University in 1975 as a postdoctoral research assistant in Microbiology to work with Professor Allan Hamilton. Sabbatical periods in Osnabruck, Chicago and San Diego, coupled with research leave fellowships from the Nuffield Foundation and The Wellcome Trust have enabled him to develop his research work on bacterial ion channels and their role in stress management in bacterial cells. He has an international reputation for the analysis of ion channels and the regulation of their gating. His research has been underpinned by Wellcome Trust Programme Funding for the last 15 years as well as by a number of different research councils. He has recently developed his interest in integrative physiology through BBSRC funding for Systems Biology of Microorganisms.
He has significant experience of national funding agendas through work with the BBSRC and The Wellcome Trust. For eight years he was a research consultant to the Upjohn Company advising on microbial processes and he has also worked with other companies in this capacity. He was appointed to a personal chair in 1989 and to the established Chair of Microbiology in 1996. He was Director of the Institute of Medical Sciences from 2005 to 2010. During his time in Aberdeen he has played significant roles in the development of the Biotechnology and Microbiology research programmes.
Until recently he was a member of the University Court and he developed a role in the commissioning of works of art for the University campus collection.
External Memberships
BBSRC Systems Biology Strategy Panel
- Research
-
Research Overview
Click on images below to view full size figures:
The growth and survival of bacteria is dependent upon the maintenance of the pH and the solute balance in the cytoplasm within an acceptable range for enzyme function and DNA integrity. For many bacteria selective gene expression and control over transport systems and channels in the cytoplasmic membrane can achieve this. Our main research interests lie in the field of homeostasis and the role of homeostatic systems in the survival of organisms subjected to stress. This encompasses research on: the regulation of cytoplasmic pH in E. coli, the gating of ion channels and regulated rtansporters and in the integration of diverse protein components into homeostatic systems. Investigations using E. coli K-12 are balanced by work with pathogens, on commensal bacteria from the gut and on isolates from patients.
 One of our major research activities is the analysis of the structure and function of bacterial ion channels and efflux systems. E. coli cells possess at least four classes of ion channels - mechanosensitive (MscL, MscS, MscM, KefA), ligand-gated (KefB, KefC), Shaker-like (Kch) and chloride (CLC). Each of these has been studied by ur group, but the major foci have been the glutathione-gated KefB and KefC systems and the mechanotransducing channels, MscS and MscK. Our major goals are to marry the genetic and physiological insights that we have gained over the last ten years with structural information through protein biochemistry and crystallography.
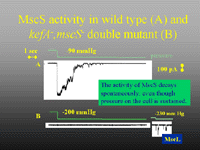
One of our major research activities is the analysis of the structure and function of bacterial ion channels and efflux systems. E. coli cells possess at least four classes of ion channels - mechanosensitive (MscL, MscS, MscM, KefA), ligand-gated (KefB, KefC), Shaker-like (Kch) and chloride (CLC). Each of these has been studied by ur group, but the major foci have been the glutathione-gated KefB and KefC systems and the mechanotransducing channels, MscS and MscK. Our major goals are to marry the genetic and physiological insights that we have gained over the last ten years with structural information through protein biochemistry and crystallography.Figure 1. Patch clamp traces for MscS and KefA (both 1 nS channels activated at similar pressure thresholds). The major channel activity seen here is MscS, which is more abundant than MscK in membrane patches and which spontaneously decays under conditions of sustained pressure.
Our group has demonstrated the role of mechanosensitive channels in cell physiology and we have developed gating models based on analysis of the structure-function analysis. MscS, the 1 nS, mechanosensitive channel and its related protein, MscK, [Figure 1] form the focus for our studies on mechanosensitive channels. A recent highlight is the determination of the structure of MscS in an open configuration in collaboration with Jim Naismith (St Andrews) [Figure 2]. By complementing this structural analysis with genetic perturbation of the system, we have been able to describe not only the structural transition between closed and open states, but also to define critical amino acids that play significant roles in the structural transition and the stability of the open state.

Our work demonstrated that KefC (and KefB) are maintained inactive by binding glutathione and are activated by glutathione adducts formed during the detoxification of electrophiles (e.g. the natural electrophile methylglyoxal). This activation leads to potassium efflux from cells that is accompanied by cytoplasmic acidification. Through a number of studies we have shown that the extent of modification of cytoplasmic pH determines the degree of protection of the DNA from electrophile-induced DNA damage. The key questions surround the mechanism of integrating detoxification with activation of the efflux system and the structure of the regulatory and efflux protein domains of KefC. Our approaches to these two questions build upon systems-based approaches to the analysis of the synthesis and breakdown of the activator and combined structural and genetic approaches to KefC. This is the basis of our current programme and this involves enzymology, structural biology and molecular genetics allied to microbial physiology.

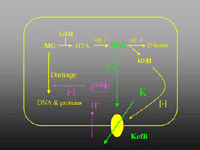
Figure 3. The basic organisation of E. coli KefB and KefC systems. Both proteins are similar in size (~ 70 kDa) and organisation (~ 380 residue membrane protein, 16-20 residue flexible region, 200 residue "soluble" domain containing a Rossman fold). The systems have an auxiliary protein YheR and YabF, for KefB and KefC, respectively, that resembles the quinone oxidoreductases. This protein is required for activity. The channels are maintained in a closed state by glutathione (GSH) and activated by glutathione adducts (GSX). When activated potassium ions leave the cell down their chemical gradient and the cytoplasmic pH is lowered due to rapid proton entry.
Figure 4. Physiological role of KefB and KefC exemplified via the detoxification of methylglyoxal (MG). MG enters the cell and reacts spontaneously with glutathione to form hemithiolacetal (HTA). Glyoxalase I converts HTA to S-lactoylglutathione, which is a strong activator of KefB. Potassium ions leave the cell and are replaced by protons and sodium ions, the consequent lowering of the pH protects the cell against the cytotoxic effects of MG.
Current Research
Our current research focusses on the structure-function relationships in ion channels and transporters and the integration of these activities to achieve cellular homeostasis.
Collaborations
Our major collaborations are with:
Jim Naismith (University of St Andrews) - structural biology of membrane protein complexes;
Stuart Conway (University of Oxford) - synthetic chemistry approaches to regulation of ion channels;
Dianne Newman (Caltech) - mechanosensitive channels and photosynthetic physiology;
Rob Phillips (Caltech) - mechanonsensitive channels and their gating and its role in cell survival
Doug Rees (Caltech) - mechanosensitive channels - structure and function
Samantha Miller (University of Aberdeen) - collaborations on microbial physiology and ion channel structure and function.
David Dryden (Edinburgh) - single cell physics;
Anca Segall (SDSU, San Diego) - inhibitors of channel and transporter activity;
Paul Blount (UTSW, Dallas) - gating of mechanosensitive channels;
Alessandro Moura (Aberdeen) - systems biology;
- Publications
-
Page 1 of 1 Results 1 to 8 of 8
Interaction of the Mechanosensitive Channel, MscS, with the Membrane Bilayer through Lipid Intercalation into Grooves and Pockets
Journal of Molecular Biology, vol. 431, no. 17, pp. 3339-3352Contributions to Journals: ArticlesAdenosine Monophosphate Binding Stabilizes the KTN Domain of the Shewanella denitrificans Kef Potassium Efflux System
Biochemistry, vol. 56, no. 32, pp. 4219-4234Contributions to Journals: ArticlesCharacterization of three novel mechanosensitive channel activities in Escherichia coli
Channels, vol. 6, no. 4, pp. 272-281Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.4161/chan.20998
Salt bridges regulate both dimer formation and monomeric flexibility in HdeB and may have a role in periplasmic chaperone function
Journal of Molecular Biology, vol. 415, no. 3, pp. 538-546Contributions to Journals: ArticlesOptimality in DNA repair
Journal of Theoretical Biology, vol. 292, pp. 39-43Contributions to Journals: ArticlesAltered antibiotic transport in OmpC mutants isolated from a series of clinical strains of multi-drug resistant E. coli.
PloS ONE, vol. 6, no. 10, e25825Contributions to Journals: ArticlesCollective response of self-organized clusters of mechanosensitive channels
Physical Review. E, Statistical, Nonlinear and Soft Matter Physics, vol. 83, no. 2, 020901(R)Contributions to Journals: ArticlesA role for mechanosensitive channels in survival of stationary phase: regulation of channel expression by RpoS
PNAS, vol. 100, no. 26, pp. 15959-15964Contributions to Journals: Articles- [ONLINE] DOI: https://doi.org/10.1073/pnas.2536607100