Targeting mechanisms of hunger and satiety, and the food-gut-brain axis, in the fight against obesity
Obesityis a major public health problem across the developed and developing world. Fundamentally, overweight and obesity is the consequence of calories ingested as food and drink exceeding those that are expended through metabolism, thermogenesis and activity. Excess calories are stored as body fat (adipose tissue). Accumulation of excess body fat is associated with metabolic diseases such as type II diabetes and cardiovascular disease that have a major impact on longevity and quality of life. Although a number of drugs have reached the market for the treatment of obesity, most of these have subsequently been withdrawn due to the emergence of unacceptable side effects. This has provided additional impetus to attempts to develop dietary strategies for the obesogenic environment, and specifically for a food solution to address the issue of over-consumption of calories and the consequences of this.
Full4Health (‘Understanding food-gut-brain mechanisms across the lifespan in the regulation of hunger and satiety for health’), is a €9 million EU Framework 7 project which brings together 19 multidisciplinary academic and industry collaborators from across Europe. The project will investigate mechanisms of hunger, satiety and feeding behaviour, and how these change across the life course, effects of dietary components and food structure on these processes, and their possible exploitation in addressing obesity, chronic disease and under-nutrition.
- Impact
-
Background
In Western Europe there has been a huge increase in the amount and variety of cheap food available to the average consumer. This sounds like good news and indeed was a situation dreamed of after years of rationing during and after World War II. However, as is so often the case, there have been some unintended consequences of this food boom. The problem lies with the increase in the variety of foods containing high levels of fat and sugar, which we find palatable and enjoyable to eat, and which are also often the cheapest to buy. Combine this with our increasingly sedentary lifestyles, and we have the perfect recipe for the increase in overweight and obesity that we see across most countries in Europe.
A different but related problem is the increasingly ageing population. Elderly people often suffer from a loss of appetite (relative anorexia), which can lead to gradual weight loss, loss of mobility and a dramatic decrease in quality of life. Inappetence is also a clinical issue in patients recovering from major traumas and chemotherapy.
The increase in overweight and obesity, and the problem of age-related and clinical anorexia are opposite ends of the same spectrum, namely, how our physiological systems interact with the food we eat and how our appetite is controlled. This is a highly complex, multi-faceted system, which involves as a first step the release of hormones from the gut in response to the consumption of food. These signals, alongside a range of other environmental and social information, are processed in the brain leading to a stimulation or reduction in appetite, and to feelings of hunger or satiation (feeling full).
Although people are often able to lose weight, they usually find it difficult to keep the weight off in the long term. This is partly because reducing food consumption is working against many of these gut-brain interactions, which have evolved to counter life-threatening weight loss and which stimulate us to eat when we see palatable food. It is also worth noting that most of the drugs that have made it into the clinic for weight loss have subsequently been withdrawn because of side-effects. So we have a significant problem of over-consumption of calories leading to overweight and obesity, without any effective long-term treatment to alleviate the condition.
Clearly, a new approach to weight control is required if we are to make any progress in the battle against obesity and improve the quality of life across a wide age range.
Full4Health outcomes and impact
The Full4Health project is undertaking an in-depth, multi-disciplinary approach to significantly improve our understanding of the pathways and mechanisms that are involved in controlling our feelings of hunger and satiety. These studies of physiological processes, and including the psychology of food choice, are complemented by studies looking at the effects of food structure and different dietary components. By combining these different approaches it is hoped to identify new food ‘leads’ for appetite control, in the same way as protein has been proven to be the most satiating macronutrient, and has recently been incorporated into several novel food products across Europe.
So in the longer term the project is aiming to provide new insights that will enable the reformulation of food and food products to provide more effective tools for appetite control. This could provide added support to a consumer-based approach to weight management, enabling individuals to take control of their health rather than relying on clinicians.
The project is clearly relevant to a range of EU health-related policies, many of which are outlined in the publication ‘Healthier Together in the European Union’ from the Health and Consumer Protection Directorate-General (ISBN 92-79-04503-2) www.bookshop.europa.eu and http://ec.europa.eu/dgs/health_consumer/index_en.htm.
In March 2005 the EU set-up the Platform for Action on Diet, Physical Activity and Health, bringing together consumer organisations, health NGOs and EU-level industry representatives to tackle the obesity problem through voluntary actions. In May 2012, Health and Consumer policy Commissioner John Dalli issued a press release emphasising the importance of the consumer ‘at the heart of the single market’. Clearly outcomes from projects such as ‘Full4Health’ should facilitate voluntary and consumer-based actions towards preventing further increases in obesity.
- Stakeholder Engagement
-
In the initial stages of the project our strategy is to meet and build relationships with a number of key policy stakeholders in the EU Commission and other relevant agencies. We will encourage project partners to undertake dissemination activities in their own regions, but we acknowledge that our biggest impact is likely to be at the EU-wide level, and so this is where we will concentrate our efforts.
We have attended key conferences to enable us to meet stakeholders, and we are using our web site and newsletters as engagement tools to keep stakeholders informed of our progress.
Engagement with the High Level Group on Nutrition and Physical Activity (European Government Representatives) and EU Platform for Action on Diet, Physical Activity and Health (European-level organisations, ranging from the food industry to consumer protection NGOs) have brought the project to the attention of a broad audience.
Subscribe to our News RSS feed or send an email to the Project Office to receive our regular Newsletters and Research Briefs.
For more information contact Dr Sue Bird
Projects
- Targeting mechanisms of hunger and satiety, and the food-gut-brain axis, in the fight against obesity
-
Obesity is a major public health problem across the developed and developing world. Fundamentally, overweight and obesity is the consequence of calories ingested as food and drink exceeding those that are expended through metabolism, thermogenesis and activity. Excess calories are stored as body fat (adipose tissue). Accumulation of excess body fat is associated with metabolic diseases such as type II diabetes and cardiovascular disease that have a major impact on longevity and quality of life. Although a number of drugs have reached the market for the treatment of obesity, most of these have subsequently been withdrawn due to the emergence of unacceptable side effects. This has provided additional impetus to attempts to develop dietary strategies for the obesogenic environment, and specifically for a food solution to address the issue of over-consumption of calories and the consequences of this.
Full4Health (‘Understanding food-gut-brain mechanisms across the lifespan in the regulation of hunger and satiety for health’), is a €9 million EU Framework 7 project which brings together 19 multidisciplinary academic and industry collaborators from across Europe. The project will investigate mechanisms of hunger, satiety and feeding behaviour, and how these change across the life course, effects of dietary components and food structure on these processes, and their possible exploitation in addressing obesity, chronic disease and under-nutrition.
Key Points
- Dietary interventions in human volunteers tend to be focussed on a specific population group, most frequently middle-aged overweight males. However, this group may not be representative of responses across the wider population where energy balance issues are important. We need to understand how responses to food, gut-brain signals, and their integration, differ across key age groups, i.e. children, adolescents, and the elderly.
- Quite a lot is known about gut-brain signalling in satiety and energy balance, but comparatively little about the modulatory role of food on these signals, and the potential of attributes of food to contribute to reduced caloric intake.
- Research on gut-brain signalling has tended to focus on the hypothalamus (the energy balance centre) and to overlook other important brain structures with integratory capability, in particular the hindbrain, where nervous and other signals from the gut converge.
Research Undertaken
The Full4Health project will integrate investigation of human volunteers and laboratory animals with emphasis on neuronal, hormonal, molecular, physiological, psychological and behavioural responses to food at different stages of the life course.
The main human dietary intervention will compare, for the first time in a single study, responses to food in 4 age groups (children, adolescents, adults and the elderly), in males and females, and in lean and overweight.
Physiological and psychological responses to food may change as we develop and age, with impact on food choices and preferences. For example, it is unknown to what extent the release of gut peptide hormones which are involved in meal-processing, but which also signal satiety to the brain, is developmentally regulated. This may be a critical issue in the battle against food intake-related chronic disease, most commonly driven by over-consumption, but also in consideration of relative under-nutrition in the elderly and clinically compromised.
The Full4Health project will examine the interaction of food and dietary components with the gastrointestinal tract, and will characterise the role of gut endocrine secretions, the vagus nerve, and hindbrain, hypothalamic and forebrain structures in signalling and integration of hunger and satiety. We will also apply imaging and other cutting edge technologies in both human
Policy implications:
Strengthened understanding of the mechanisms of hunger and satiety, and the potential to manipulate these mechanisms through the diet has direct relevance to any strategy to prevent obesity and overweight, since these conditions are primarily driven by over-consumption of calories.
The behavioural, psychological and mechanistic data generated following the stratification of the general population into age groups will also relate to policy strategies being pursued to promote healthier childhood and healthy ageing, key issues in view of the alarming progress of childhood obesity and the projected national demographic.
Additionally, there is potential to grow the food and drink sector by developing diets, foods or supplements that exploit the role of food in the food-gut-brain axis to promote healthier lives.
- Dietary interventions in human volunteers tend to be focussed on a specific population group, most frequently middle-aged overweight males. However, this group may not be representative of responses across the wider population where energy balance issues are important. We need to understand how responses to food, gut-brain signals, and their integration, differ across key age groups, i.e. children, adolescents, and the elderly.
- Hunger hormones
-
Can the body’s hunger hormones be manipulated to promote weight loss?
- Hunger is presumably one of the earliest sensations we experience. The sensations of hunger and fullness (or satiety), are complex and occur in response to neural, hormonal, habitual and social cues. Researchers at the Institute of Metabolic Science in the University of Cambridge are particularly interested in the hormonal regulation of hunger and fullness, especially regarding the role of the hormone glucagon-like peptide 1 (GLP-1). GLP-1 is made in the lower small intestine and promotes a feeling of fullness. It is produced by specialised gut cells in response to nutrients in the gut, such as might occur after a meal. Concentrations of GLP-1 increase markedly after bariatric surgery and this increase may be in part responsible for the weight loss and improved blood sugar control seen in people after bariatric surgery.
- Full4Health partners Fiona Gribble, Claire Meek and colleagues at the University of Cambridge carried out a study to identify if they could promote fullness using a nutritional product to increase levels of GLP-1, to help people lose weight without the need for surgery. The nutritional product was delivered in a specialised capsule which was designed to release its contents close to the gut cells where GLP-1 is produced. They used three different types of nutritional products containing either protein, fat or a cholesterol derivative (bile acid). Healthy volunteers and people with type 2 diabetes were invited to attend the Clinical Research Facility at Addenbrooke’s hospital to ingest the capsules and have blood taken. They also assessed the effects of the capsules upon hunger, blood glucose levels and meal size.
What did they find?
- The protein and fat supplements did not appear to work well and did not increase the level of GLP-1 in the blood or affect hunger or satiety. However, the cholesterol derivative showed some initially promising results. Unfortunately, this substance was relatively unstable inside the capsules and had a short shelf life which limited the amount of testing they could perform. They are now thinking of other ways to use bile acids to stimulate GLP-1 production.
- Obesity is a global problem: tackling obesity with cost-effective and non-invasive measures remains a priority. The aim of this study was to test a potential option for promoting fullness and reducing hunger to help people attain a healthy weight – a potential alternative to bariatric surgery. Although none of these capsules were suitable for further testing, it was discovered that it is safe, feasible and convenient to deliver nutrients in this way to human volunteers. The research also suggested that the bile acids deserve more investigation in the future, although an alternative delivery method may be needed to increase the product’s stability.
The effect of encapsulated glutamine on gut peptide secretion in human volunteers
Claire L. Meek, Hannah B. Lewis, Bensi Vergese, Adrian Park, Frank Reimann, Fiona Gribble
Peptides 77: 38-46 - Hunger is presumably one of the earliest sensations we experience. The sensations of hunger and fullness (or satiety), are complex and occur in response to neural, hormonal, habitual and social cues. Researchers at the Institute of Metabolic Science in the University of Cambridge are particularly interested in the hormonal regulation of hunger and fullness, especially regarding the role of the hormone glucagon-like peptide 1 (GLP-1). GLP-1 is made in the lower small intestine and promotes a feeling of fullness. It is produced by specialised gut cells in response to nutrients in the gut, such as might occur after a meal. Concentrations of GLP-1 increase markedly after bariatric surgery and this increase may be in part responsible for the weight loss and improved blood sugar control seen in people after bariatric surgery.
- Gut hormone responses
-
Weak gut hormone responses to food could explain why some people don’t lose fat with exercise training.
- Exercise training gives rise to a variable degree of body weight and fat mass loss, and is associated with individual differences in appetite control (subjective hunger, food choice and energy intake). Gut hormones released during and after eating are known to influence appetite but their role in exercise-induced compensatory eating was previously unknown.
- Full4Health partners, Graham Finlayson, John Blundell and Catherine Gibbons from University of Leeds investigated the role of postprandial (post-meal) peptides (total and acylated ghrelin, insulin, CCK, GLP-1 and PYY) in overweight and obese men and women during 12-weeks supervised exercise.
- Exercisers were classified as Responders (expected fat loss) or Non-Responders (less than expected fat loss) according to measured body composition changes. A control group of non-exercisers were measured over the same time-course.
- Despite no differences in body weight or fat mass between Responders and Non-Responders before exercise training, Responders showed a greater postprandial suppression of acylated ghrelin along with higher levels of GLP-1 and PYY both before and after exercise.
- Thus postprandial gut hormone responses appear to form part of the pre-existing physiology of exercise Responders compared to Non-Responders and may explain differences underlying exercise-induced compensatory eating.
- Physical inactivity and overconsumption of calories/food are independent risk factors for morbidity and mortality. Exercise is an essential part of a healthy lifestyle. Understanding the relationship between physical activity, overconsumption and obesity for a range of health outcomes is critical.
- Conference abstract submitted to International Congress on Obesity, Vancouver, May 2016
Authors: C Gibbons, P Caudwell, D-L Webb, P Hellstrom, E Naslund, J Blundell and G Finlayson
- Workpackages
-
A food solution to obesity?
Full4Health is an EU funded project which investigates how the brain and the gut interact to control hunger, satiety ("feeling full") and feeding behaviour, and how this changes across the life course. Understanding the effects of dietary components and food structure on these processes may help to develop food based products to address obesity, chronic disease and under-nutrition.
The vision is that we can generate evidence-based recommendations about food composition that makes us feel full while reducing overall calorie intake. By working with partners from the food industry, we will accelerate the translation of understanding into practical solutions. Exploiting natural mechanisms of hunger and satiety would have many advantages over a pharmaceutical approach.
Objectives
Full4Health has been awarded 9 Mill Euros by the European Commission to meet the following objectives in the years 2011-2016.
Objective 1: To identify and characterise the neural circuits and molecular mechanisms that are integrated in the central (brain) regulation of hunger, satiety and feeding behaviour.
There is poorer understanding of the response of hindbrain structures to food-related signals, the relative importance of blood-borne vs neural (vagal) signalling in the mechanism of hunger/satiety, the potential to manipulate these signals, and identify dietary components/food structure that can help to control food intake.
Objective 2: To investigate the integration of food-related, gut hormone and neural (vagal) signals in hindbrain relay structures and their role in hunger and satiety.
There is poor understanding of the mechanisms by which nutrients arriving in the intestinal tract act to release the gut hormones that contribute to satiety.
Objective 3: To establish the impact of bioactive compounds from food or gut origin on synthesis and secretion of gut hormones relevant to food intake and energy balance, and underlying molecular mechanisms.
We do not know how the diet/microbiota interaction influences the balance between hunger and satiety, and the activity of gut hormone and brain peptide signalling systems.
Objective 4: To determine the impact of the gut microbiota in relation to food composition on central mechanisms of hunger and satiety.
Little is known of the role of gut-derived signals in the nutritional programming of brain circuits involved in feeding and body weight regulation - disruptions in gut-brain communication during critical periods of life may prompt substantial changes in the development of hunger and satiety networks.
Objective 5: To investigate the involvement of early post-natal nutrition on the development of gut-brain signalling systems, and the impact of hypothalamic sensitivity to gut-derived signals on life-long feeding behaviour and energy balance.
The psychological, behavioural and endocrine/neurological bases of these effects and their applicability across age, gender and body phenotype remain to be determined. We do not understand the variability in psychological and behavioural parameters of hunger/satiety and food preference during energy deficit (exercise or diet induced) across the life course, how these manipulations relate to gut hormones, neural
activation and energy metabolism, or how these responses might vary in European populations.
Objective 6: To relate psychological and behavioural parameters of hunger/satiety and food preference to gut hormones, neural activation and energy metabolism during energy deficit and dietary manipulation, and across the lifespan.
The role of protein has largely been ignored. Intake of protein appears to be very tightly regulated in laboratory rodents, but we do not know whether dietary protein also exerts leverage to drive food and energy intake in humans,but we do not know whether dietary protein also exerts leverage to drive food and energy intake in humans.
Objective 7: To establish the relative importance of dietary protein content in determining energy intake.
- Full4Health Science News
-
Effect of hunger hormone in early life may influence obesity risk in later life
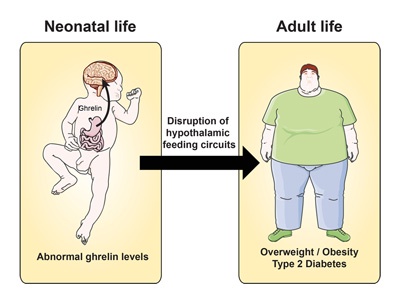
- A complex network of neural circuits regulates body weight and energy balance. When the stomach is empty, ghrelin is secreted and acts on the arcuate nucleus in the brain to initiate feeding. Until now, little was known about the importance of ghrelin on development of brain mechanisms regulating body weight and appetite.
- Full4Health partner, Sebastien Bouret and his team in Lille investigated the role of ghrelin during early life in mice:
- Blocking the hormone soon after birth resulted in more axonal projections in the arcuate nucleus and caused lifelong metabolic disturbances, including obesity and diabetes.
- Increasing ghrelin during this period impaired the normal growth of arcuate projections and caused metabolic dysfunction.
- Thus neonatal ghrelin directly influences development in the part of the brain related to appetite and the regulation of metabolism.
-
Sufferers of Prader-Willi syndrome exhibit elevated ghrelin levels. Uncontrolled hunger in these patients leads to severe obesity so a greater understanding of the role of ghrelin in early life will be important in development of interventions to reverse the symptoms of metabolic disease.
J Clin Invest. 2015 Feb; 125(2):846-58. doi: 10.1172/JCI73688
