PhD-students currently working in this area:

We work on the distribution of inorganic arsenic in different food and feed-stuff. One of the major areas we work on together with other research groups is the determination of inorganic arsenic in rice grown under different conditions.
This led in 2015 to the introduction of maximum permissible levels of inorganic arsenic in rice products in the EU and our Feature Article on the analytical aspects of arsenic speciation in food and feed legislation
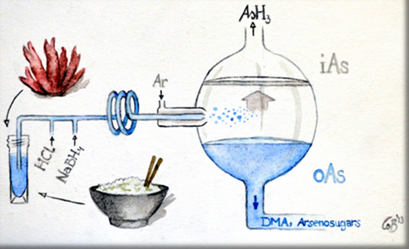
- Musil et al. Anal Chem 2014, Petursdottir et al. Anal. Met. 2014)
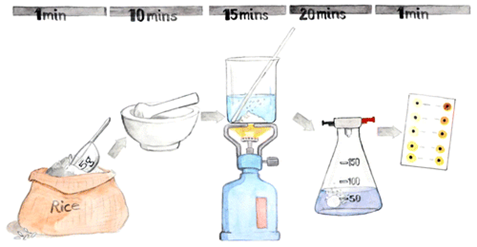
To allow on-site measurement of inorganic arsenic in rice and seaweed a field-deployable method suitable for use without sophisticated lab-based analytical instrumentation was developed and tested.
- (Bralatei et al. Anal. Chem. 2015, Bralatei et al. Microchim Acta 2017)
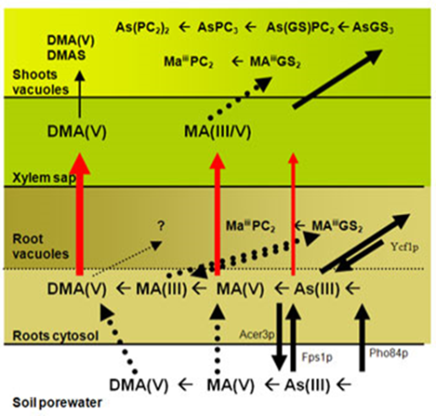
We also work(ed) on the uptake and metabolism of inorganic arsenic, selenium and mercury by plants generally. Experiments showed that plants store / transport inorganic arsenic generally as peptide-complexes bound to sulphur.
The proportion of elements complexed and the exact species of these complexes vary by elements and plant species. Of particular interest is the translocation of arsenicals in plants and the role of arsenic phytochelatins in this since this often determines the arsenic content in the edible plant part(s).
- (e.g., Batista et al. J. Exp. Bot. 2014)
Terrestrial plants do not normally produce organic arsenic compounds from inorganic arsenic in contrast to algae, especially marine macroalgae (seaweed). These not only enrich arsenic in their tissue, but they actively metabolise inorganic arsenic to a wide variety of organo-arsenicals some of which are water-soluble and some lipid-soluble. (Raab et al. Anal. Chem. 2013, Petursdottir et al. Environ. Chem. 2015)
Seaweed is a good fertiliser and considered the vegetable of the 21st century. But was it used already by the Neolithic people? Investigations into metal fingerprinting and stable isotope signatures for seaweed fertilization are currently researched in collaboration with
- Dr Ingrid Mainland – UHI
- Mark Taggart - ERI
- Dr Philippa Ascough - University of Glasgow
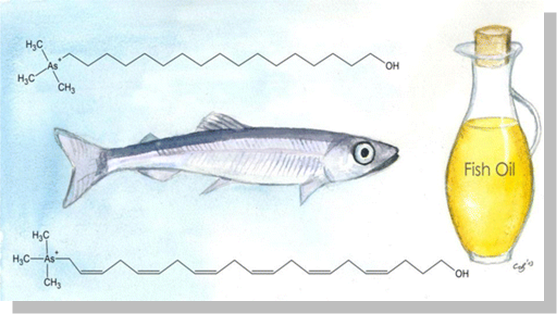
Fish also contain arsenolipids whose toxicity seems to be more complicated with some compounds nearly as toxic as inorganic arsenic.
The method development for quantification and structure elucidation of lipid-soluble arsenic is an ongoing research field and since it is non- targeted analysis we describe this as arsenomics. (e.g., Amayo et al. Anal. Chem. 2011, 2013, Petursdottir et al. Environ. Chem. 2015, Pereira et al. JAAS 2016)

Most of the arsenic is excrete in organic form, either as taken-up (arsenobetaine) or metabolised to an organo-arsenical via urine. The non-excreted arsenic is enriched in skin, hair and nail. Urine can be used as a monitor for recent exposure, whereas hair, skin and nails are indicating past exposure which makes them interesting markers also for archaeologists studying possible past exposure (Raab & Feldmann ABC 2006).
However, external post-mortem contamination makes the interpretation often difficult. Hence, arsenic speciation analysis which can determine arsenic metabolites could give the real exposure to arsenic. (Perry et al. PNAS 2013, Lancet 2015, PLoS Negl. Trop. Dis. 2015).
Recently, we have been awarded CAPABLE, a GCRF funded collaboration between University of Cambridge, UCL, icddr,b, National Heart Foundation Bangladesh, BSMMU, IEDCR.

Volatile arsenic compounds are also a problem for the oil and gas-industry. (Krupp et al. Spectrochim. Acta 2007, Uroic et al. JEM 2009)
To enable on-site collection of volatile arsenic a chemotrapping method was developed allowing the determination of the exact arsenic species in the original sample without necessitating the transport of gaseous samples from the field into the laboratory.
(Mestrot et al. ES&T 2009, 2011, Mestrot et al. Environ. Sci.-Proc. Imp. 2013)
Some of our publications in this field:
- Speciation and localization of arsenic in white and brown rice grains
- Detection of Inorganic Arsenic in Rice Using a Field Test Kit: A Screening Method
- Uptake and translocation of inorganic and methylated arsenic species by plants
- Identification and Quantification of Arsenolipids Using Reversed-Phase HPLC Coupled Simultaneously to High-Resolution ICP-MS and High-Resolution Electrospray MS without Species-Specific Standards
- Dermal Uptake of Arsenic through Human Skin Depends Strongly on Its Speciation
- Chemotrapping-atomic fluorescence spectrometric method as a field method for volatile arsenic in natural gas
- Field Fluxes and Speciation of Arsines Emanating from Soils
-
