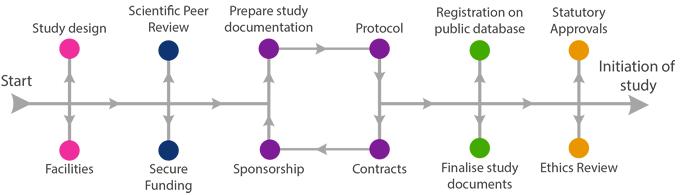
Study Design
Successful studies rely on good preparation.
Consider trial design before developing your protocol. Identify all the practical requirements.
- Is your proposal relevant to the patient population?
- Can you get Patient and Public Involvement (PPI)? - Many funders now require genuine involvement as a condition of funding as growing evidence suggests that PPI has a positive impact on both recruitment and retention in clinical trials
- Have you chosen the appropriate outcome(s)
- What is the intervention?
- When and for how long?
- If you are using a drug, how will you obtain this?
- Realistically consider if you will be able to recruit your recruitment target
Contact Pharmacy for:
- Budgeting for IMP costs
- Help to ensure you comply with the regulations required for IMP management
- Storage of drug
Contact Statistician for:
- Justification of sample size
- Randomisation requirements
- Statistical analysis plan
Contact Data Management for:
- Help with the design of Case Report Form (to collect data for your outcomes)
- Design of database
- Help with a website
Contact Clinical Trials Unit for:
- Randomisation
- Trial management advice
Identifying all practical requirements early will assist in preparing a research grant application.
Go to the SOPs and Templates page for all SOPs relevant to setting up and managing a clinical study sponsored by NHS Grampian and/or the University of Aberdeen.